The hepatitis C treatment pipeline
14 July 2011. Related: Pipeline report, Hepatitis coinfection.
Tracy Swan
Special thanks to Juliana Chan and Polly Clayden
Dedicated to Luis Mendão: brilliant activist and delightful human being.
The thrill is already gone
In May of 2011, a long-awaited improvement in the standard of care for hepatitis C virus (HCV) became reality: the US Food and Drug Administration (FDA) approved the first direct-acting antivirals (DAAs), Merck’s boceprevir (Victrelis) and Vertex’s telaprevir (Incivek). But excitement about these new drugs has already been overshadowed by a triple-whammy: the first proof-of-concept that hepatitis C can be cured without peginterferon (PEG-IFN) and ribavirin (RBV); reports of better drugs on the horizon; and challenges in clinical care, including the complexity and cost of new HCV regimens, a critical shortage of specialists to administer HCV treatment, and lack of infrastructure for treatment delivery.
Evolution to revolutionary?
In April 2011, groundbreaking results from a 21-person pilot study were announced: after only 24 weeks of treatment with two oral DAAs, a protease inhibitor and an NS5a inhibitor from Bristol-Myers Squibb, four of ten people were cured. Quad therapy (with peginterferon and ribavirin added) was even more effective, curing nine of ten people.1
For now, the success of HCV treatment rests largely upon response to peginterferon. Hopefully, peginterferon will become a therapeutic relic as the standard of care for HCV continues to evolve. The next batch of DAAs may cure more people in less time than triple therapy with peginterferon, ribavirin, and boceprevir or telaprevir. Trials are exploring DAA combinations without peginterferon; some are using it only when DAAs do not fully suppress HCV. Although ribavirin plays an essential role at present, there may be equally effective and more tolerable replacements in the future.
Clinical issues in the United States
“The availability of DAA therapy will forever change the landscape of HCV, in that we will be able to cure patients of disease who we were unable to cure in the past. Unfortunately, this medical breakthrough will be coupled with resource scarcity… The influx of patients requesting HCV therapy will present a significant problem…On average, a health care provider can reasonably initiate therapy on only three patients each week before exceeding their work capacity… we anticipate at least 500 requests for evaluation for HCV therapy within the first few weeks of DAA availability, [so] current staffing will be unable to meet the demands of all patients with HCV.”
Andrew Aronsohn and Donald Jensen,’Distributive Justice and the Arrival of Direct-Acting Antivirals: Who Should Be First in Line?’
The current challenge – to fully realise the benefits of boceprevir and telaprevir by avoiding drug resistance and treatment failure – is immediately ahead of us. In the United States, there are not enough specialists to meet current demand for HCV treatment. Many have ?warehoused? patients in anticipation of better treatment, and are not seeing any new patients. At the same time, patient management is becoming more complex, involving response-guided therapy and drug-specific treatment algorithms. We are not prepared for the anticipated surge in HCV treatment uptake triggered by more effective treatment.
Access issues
“…And, please, let me talk about money. In Spain, we received the news about the price of boceprevir and telaprevir in the US with incredulity. We are indignant, and we do not understand how pharmaceutical companies can justify these outrageous prices. Western society has given the private sector the opportunity to research and develop treatment, but this doesn?t entitle you to charge disproportionate prices. At a time when cuts are causing the closure of entire hospital units, emergency rooms and a variety of medical services in this country, how do you expect us to be able to advocate for your new drugs?”
Xavi Franquet, European Community Advisory Board and Grupo de Trabajo sobre Tratamientos del VIH, Sitges IV Meeting, June 2011
Most of the 130 to 170 million people who are living with chronic hepatitis C will not be cured, because HCV treatment is too expensive. Many high-burden countries cannot afford to offer hepatitis C testing, let alone treatment. Although efforts to produce generic peginterferon are underway, access to HCV treatment remains limited or nonexistent. In the United States, 48 weeks of peginterferon and ribavirin costs more than $30,000. Boceprevir-and telaprevir-based regimens halved treatment duration for 44% (boceprevir) to 58% (telaprevir) of study participants 2,3,4. But any possible savings on peginterferon and ribavirin are offset by the cost of HCV protease inhibitors (see Table 1. Cost of HCV treatment with a protease inhibitor in the United States).
Diagnostics
Unfortunately, the boom in drug development has not been accompanied by innovative and affordable diagnostics for HCV, and point-of-care viral load testing for monitoring response to treatment. There is no single test for acute-stage HCV, and diagnosis of chronic hepatitis C remains an expensive two-step process. In many parts of the world, people do not have access to HCV testing. Even when HCV RNA testing is accessible, the process can be overly cumbersome, since people have to return for a second testing visit without being given a diagnosis. Research to streamline HCV diagnostics with a single inexpensive test should proceed in tandem with drug development.
Table 1. Cost of HCV treatment with a protease inhibitor in the United States
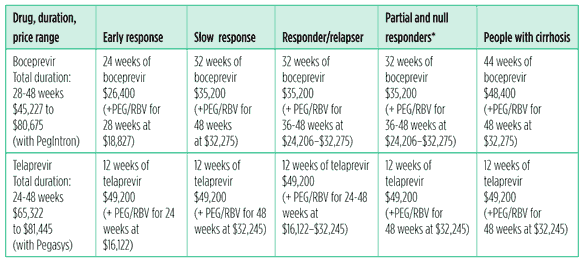
(Does not include: HCV RNA testing and other labs, medical visits, and additional medications for side effects)
*Boceprevir labeling suggests that people with <0.5 log 10 drop in HCV RNA at week 4 are likely to be null responders; they were not included in the phase III trial for treatment-experienced people.
Public and private payers are sure to balk at the cost of DAAs, and are likely to impose restrictive treatment eligibility criteria and other barriers, such as prior authorization and top-tier pricing, making access difficult. It is unfortunate that payers are the strongest recourse for price controls.
Research issues
HCV clinical trials are going to become even more complex, with the advent of triple therapy (peginterferon and ribavirin plus boceprevir or telaprevir). Different dosing schedules will make it difficult to assess efficacy of a single drug and to compare regimens. Boceprevir and telaprevir need to be taken every eight hours, while most second-generation DAAs are once-a-day drugs. Blinded trials will require twice-daily placebo with once-daily drugs. People who are taking a once-daily drug plus placebo may skip their only dose of active drug, placing them at higher risk for treatment failure and drug resistance, since adherence is known to worsen with more frequent dosing requirements. The advantages of a once-daily drug may be obscured by placebo.
HCV clinical trials must incorporate drug- and patient-specific considerations. Treatment algorithms and stopping rules differ for each drug and according to the population it is studied in. Host and viral factors, such as IL28B genotype, stage of liver disease, race/ethnicity, age, HCV subtype (1a versus 1b), and prior response to HCV treatment must also be taken into account. Designing clinical trials and interpreting their results will become more and more of a challenge.
Cure rates with telaprevir- and boceprevir-based regimens are high, making it more difficult for other DAAs to demonstrate superiority. Non-inferiority trials will be needed for the next generation of drugs. These agents are likely to offer other advantages, such as shortened treatment duration, simpler regimens, and more convenient and tolerable drugs. Hopefully regulators and sponsors will consider non-traditional endpoints, such as treatment duration and discontinuations for adverse events, and type, incidence and severity of side effects, along with efficacy. Tolerability and convenience are also extremely important to people who will be taking these drugs.
Plea for simplicity
Interpreting data from complex clinical trials and translating them directly into clinical practice is difficult, particularly in the absence of a standing, multidisciplinary treatment guidelines panel, an approach that has optimised HIV treatment outcomes and facilitated reimbursement by public and private payers. Simplicity should become a major focus of HCV drug development.
Most need, least data
Merck and Vertex chose not to conduct early access trials in people with urgent need, who were ineligible for their clinical trials. Early access trials could have saved lives, and allowed physicians to gather information about if and how HCV protease inhibitors could be used in patients who need them most. It is likely that boceprevir and telaprevir will be used in desperate patients, regardless of the lack of information about their safety and efficacy. It is time for the pharmaceutical industry to work with regulators, physicians, and activists to launch early access trials.
Unfortunately, boceprevir and telaprevir are not going to be able to get the job done for people with poor prognostic factors, urgent need, and peginterferon intolerance. Prior null responders with cirrhosis did not reap much benefit; adding telaprevir to peginterferon and ribavirin increased the cure rate from 10% to only 14%, and there are limited data on boceprevir in this population.5
Safety and efficacy of boceprevir and telaprevir are not yet known in HIV/HCV coinfected people (although pilot studies are ongoing, and larger ones planned). No studies have been initiated in children, the elderly, people with renal insufficiency, or liver transplant candidates and recipients (although there have been no pharmacokinetic studies of boceprevir and telaprevir is not recommended for people with hepatic impairment).
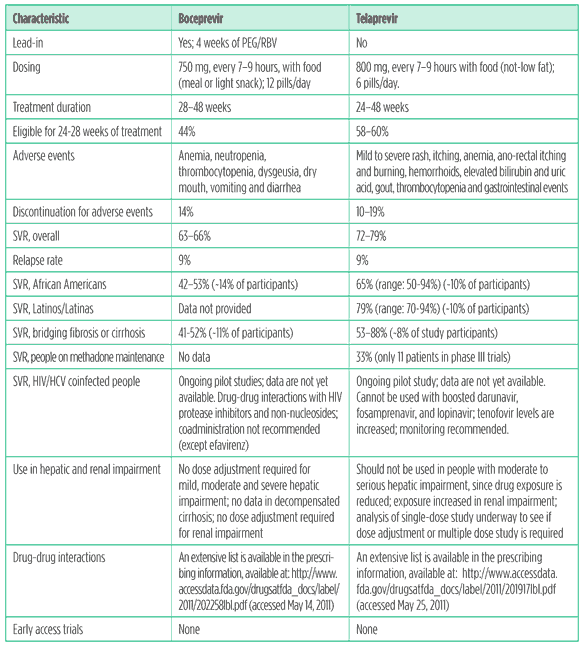
Despite low enrollment of African Americans in registration trials for boceprevir and telaprevir, it is clear that adding one of these drugs significantly increased rates of sustained virological response (SVR; meaning no hepatitis C can be detected six months after treatment completion; regarded as a cure) over PEG-IFN/RBV (see Table 2. Translating trial results into clinical practice: boceprevir and telaprevir in treatment-naive persons). But there are lingering questions about the optimal duration of boceprevir-based therapy in African Americans, since the SVR among African Americans was 11% lower for response-guided therapy versus 48 weeks of treatment.3
Unfortunately, data on SVR among Latinos/Latinas are scarce. Latinos and Latinas comprised less than 10% of treatment-naive study participants in telaprevir phase III trials Nonetheless, telaprevir did boost SVR among treatment naive Latinos/Latinas; SVR rates ranged from to 70% to 94%, a significant improvement over peginterferon and ribavirin.2,4 Merck did not provide any data on boceprevir in Latinos and Latinas.
Drug-drug interactions
HCV protease inhibitors share metabolic pathways with medications that are commonly used by people with hepatitis C. Drug-drug interactions can lead to drug resistance and HCV treatment failure when levels of an HCV protease inhibitor are too low, or worsen side effects when they are too high. In turn, HCV protease inhibitors may decrease levels of other drugs to sub-therapeutic levels, or increase them to toxic levels.
Interactions between antiretroviral agents and DAAs complicate HIV treatment; a single drug or entire regimen may need to be switched before initiating hepatitis C treatment. This is tricky, because HIV/HCV coinfected people have limited options for treating their HIV while on HCV treatment; in the ongoing telaprevir coinfection trial, participants could either use an efavirenz-based regimen (with a higher dose of telaprevir) or a boosted atazanavir-based regimen (other HIV protease inhibitors cannot be used with telaprevir, due to significant drug-drug interactions).6 Unfortunately, data on boceprevir drug-drug interactions are limited to efavirenz (which lowers boceprevir levels) and tenofovir.7
Since hepatitis C is highly prevalent among current and former injection drug users, drug-drug interaction studies with DAAs and opioid substitution therapy (OST) must be a priority. If DAAs are used in people on OST without this information, a range of consequences may occur, including HCV drug resistance and treatment failure, drug overdose, or withdrawal symptoms. So far, it has been established that telaprevir reduces methadone levels; although dose adjustment may not be needed, clinical monitoring is recommended. A drug-drug interaction study of telaprevir and buprenorphine is underway. Unfortunately, there are no drug-drug interaction data on methadone and buprenorphine for boceprevir, save the warning in the label, which reads “Plasma concentrations of methadone or buprenorphine may increase or decrease when coadministered with VICTRELIS [boceprevir’s brand name]. However, the combination has not been studied. Clinical monitoring is recommended as the dose of methadone or burenorphine may need to be altered during concomitant treatment with VICTRELIS.”
Current research landscape
Are you experienced?
The FDA has categorised treatment-experienced people into three groups:
- Responder-Relapser: HCV RNA undetectable at end of treatment with peginterferon and ribavirin, but HCV RNA detectable within 24 weeks of treatment follow-up.
- Partial Responder: greater than or equal to 2 log10 (99%) reduction in HCV RNA at week 12, but not achieving HCV RNA undetectable at end of treatment with peg-interferon and ribavirin.
- Null Responder: less than 2 log10 (99%) reduction in HCV RNA at week 12 of treatment with peginterferon and ribavirin.
People who could not tolerate treatment or do not know their prior response are not included; nor are people who experienced viral breakthrough during treatment.
There are dozens of HCV clinical trials underway, but most are for treatment-naive participants. Of the nine phase II/III HCV protease inhibitor trials, six are open to treatment-experienced participants (some may be limited to responder-relapsers and partial responders). Current phase II polymerase and NS5a inhibitor trials offer few options: treatment-experienced participants are eligible for only one of three NS5a inhibitor trials, one of two nucleoside/nucleotide polymerase inhibitor trials, and one of seven non-nucleoside polymerase inhibitor trials.
At present, an estimated 750,000 people in the United States (and thousands more in Western Europe) have been unsuccessfully treated for hepatitis C. Treatment-experienced patients are likely to be first in line for treatment with new HCV drugs; financial experts project that by 2013 they will comprise at least two-thirds of the market share for DAAs. The population of treatment-experienced people is large, and will continue to grow once the first generation of hepatitis C protease inhibitors have been widely used. More trials exploring DAA combinations and treatment strategies are needed for treatment-experienced people.
Multi-DAA/quad therapy trials will help to clarify the best approach based on HCV subtype, treatment history and other factors. Abbott, Boehringer Ingelheim, Bristol-Myers Squibb (BMS), Genentech, Gilead, Merck, Pharmasset, and Vertex have opened in-house combination trials.
A single company may not have all of the most exciting drugs, so cross-company clinical collaborations are needed to optimise HCV treatment. BMS and Pharmasset, who have very exciting DAA candidates, launched the first cross company clinical collaboration; in May of 2011, they announced a peginterferon-sparing trial combining BMS’s NS5a inhibitors with one of Pharmasset’s nucleotide polymerase inhibitors (PSI-7977), with or without ribavirin. These joint ventures are crucial for identifying best-in-class regimens and optimising HCV treatment.
Boceprevir and telaprevir
Improvements in HCV treatment (such as response-guided therapy and drug- and patient-specific treatment algorithms) bring a new level of complexity to clinical care, making it unappealing to inexperienced providers. During FDA approval hearings for boceprevir and telaprevir, an antiviral advisory committee member remarked that the amount of knowledge required to treat HCV approaches the complexity – if not the wisdom – of a Talmudic scholar. Complexity brings consequences, such as greater potential for errors in prescribing and administering treatment, higher dropout rates from poorly managed side effects, and increased risk for drug resistance and treatment failure.
Clinicians and their patients will have a choice between boceprevir and telaprevir-based treatment. Although they are in the same class, these drugs are used differently. Boceprevir requires a four-week ‘lead-in’ with peginterferon and ribavirin to lower on-treatment failure and relapse rates; telaprevir is initiated along with peginterferon and ribavirin. With each drug, there may be a peginterferon and ribavirin ‘tail’ after triple therapy, lasting 12 to 36 weeks.
Telaprevir offers treatment-naive patients a less complex treatment algorithm, higher cure rates, and a better chance to shorten treatment than boceprevir, but tolerability may be an issue. More than half of the participants in all phase III trials were afflicted with rash (versus 32% for PEG/RBV alone). Although most cases were mild-to-moderate, 1% suffered severe rash, and a subset of these cases (<1%) experienced Stevens Johnson Syndrome (SJS, a rare, life-threatening reaction to a medication or infection) or Drug Related Eruption with Systemic Symptoms (DRESS, another severe drug reaction).
Boceprevir worsens the hemotologic side effects of peginterferon and ribavirin, especially anemia. During phase III trials, more than 40% of participants in the boceprevir arms were treated with epoetin alfa, a red blood growth cell factor.3,8 Red blood cell growth factors carry a warning about increased mortality, serious cardiovascular and thromboembolic events, stroke and risk of tumor progression or recurrence in cancer patients. They are also expensive, adding at least $500 per week to the cost of HCV treatment. Anemia can also be managed by reducing the dose of ribavirin, but this strategy may reduce treatment efficacy; an ongoing trial of boceprevir-based treatment is comparing ribavirin dose reduction to epoetin alfa use.
Table 2. Translating trial results into clinical practice: boceprevir and telaprevir in treatment-naive persons
Sources:
FDA briefing documents for boceprevir and telaprevir: available at http://www.fda.gov/downloads/AdvisoryCommittees/ CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252341.pdf and http://www.fda.gov/downloads/Advisory Committees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252561.pdf (accessed May 26, 2011)
JacobsonIM,McHutchisonJG,DusheikoG,etal;ADVANCEStudyTeam.Telaprevirincombinationwith peginterferonalfa-2aand ribavirin in genotype 1 HCV treatment naive patients: final results of phase III ADVANCE study. [abstract LB-2] 61st Annual Meeting of the American Association for the Study of Liver Diseases. Boston, Massachusetts. October 29-November 2, 2010.
Poordad F, McCone J Jr, Bacon BR, et al; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1195-206.
Sherman KE, Flamm SL, Afdhal NH, et al; ILLUMINATE Study Team. Telaprevir in combination with peginterferon alfa-2a and ribavirin for 24 or 48 weeks in treatment-naive genotype 1 HCV patients who achieved an extended rapid viral response; final results of phase III ILLUMINATE study. (Abstract LB-1) 61st Annual Meeting of the American Association for the Study of Liver Diseases. Boston,Massachusetts.October 29-November2,2010.
Fig 1. Boceprevir: treatment naive algorithm
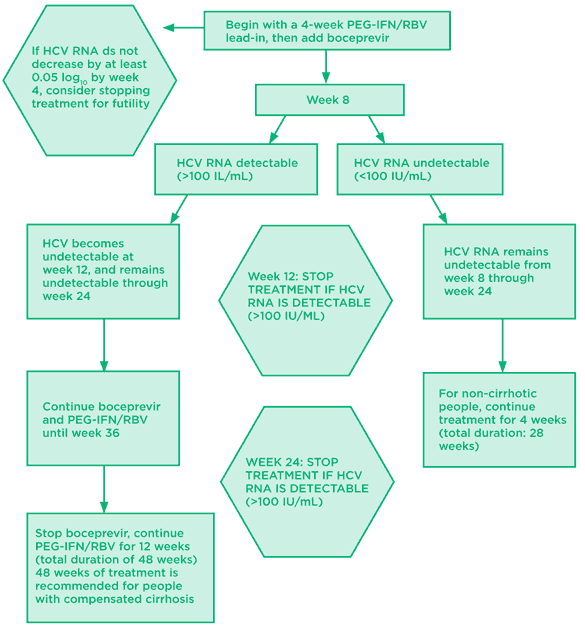
Source: Prescribing information for boceprevir. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202258lbl.pdf (accessed May 25, 2011).
Fig 2. Telaprevir: treatment-naive algorithm
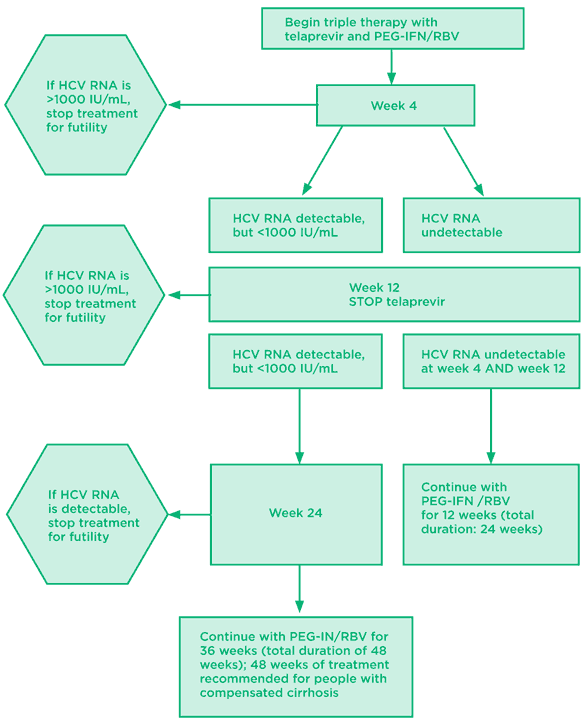
Table 3. Translating trial results into clinical practice: boceprevir and telaprevir in treatment-experienced persons

Sources:
Prescribing information for boceprevir and telaprevir. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202258lbl.pdf and http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201917lbl.pdf (accessed May 25, 2011).
Bacon BR, Gordon SC, Lawitz E, et al; HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1207-17.
Zeuzem S, Andreone P, Pol S, et al. REALIZE trial final results: telaprevir-based regimen for genotype 1 hepatitis C virus infection in patients with prior null response, partial response, or relapse to peginterferon/ribavirin (Opening and General Session 1) 46th Meeting of the European Association for the Study of the Liver. Berlin, Germany. March 30-April 3, 2011.
Fig 3. Boceprevir: treatment-experienced algorithm
Data from prior responder-relapsers and partial responders; null responders excluded
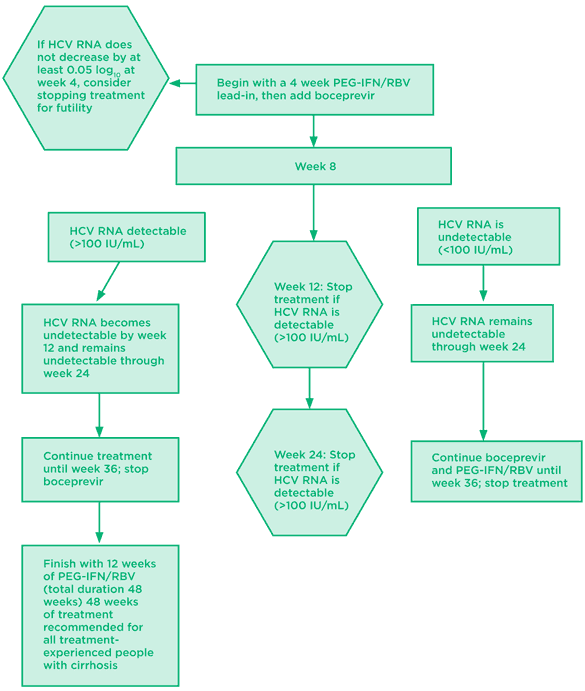
Source: Prescribing information for boceprevir. Available at:
http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202258lbl.pdf (accessed May 25, 2011).
Fig 4. Telaprevir: treatment experienced algorithm*
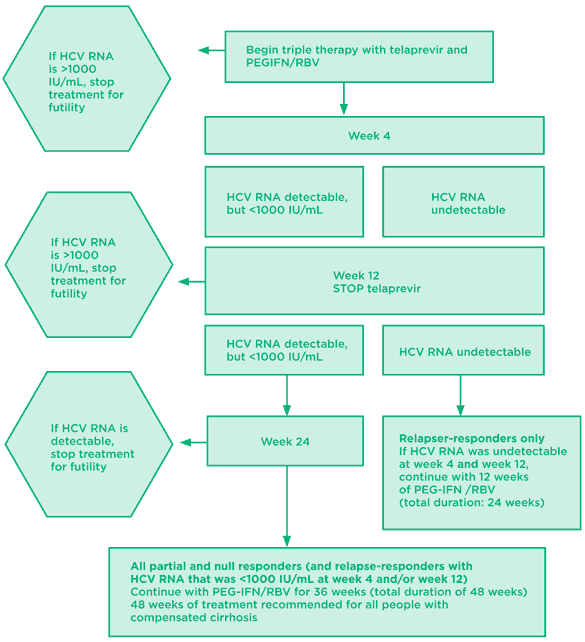
*Telaprevir labeling notes that a high proportion of null responders, especially those with cirrhosis, did not achieve SVR and developed drug resistance.
Source: Prescribing information for telaprevir. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201917lbl.pdf (accessed May 25, 2011)
HCV protease inhibitors
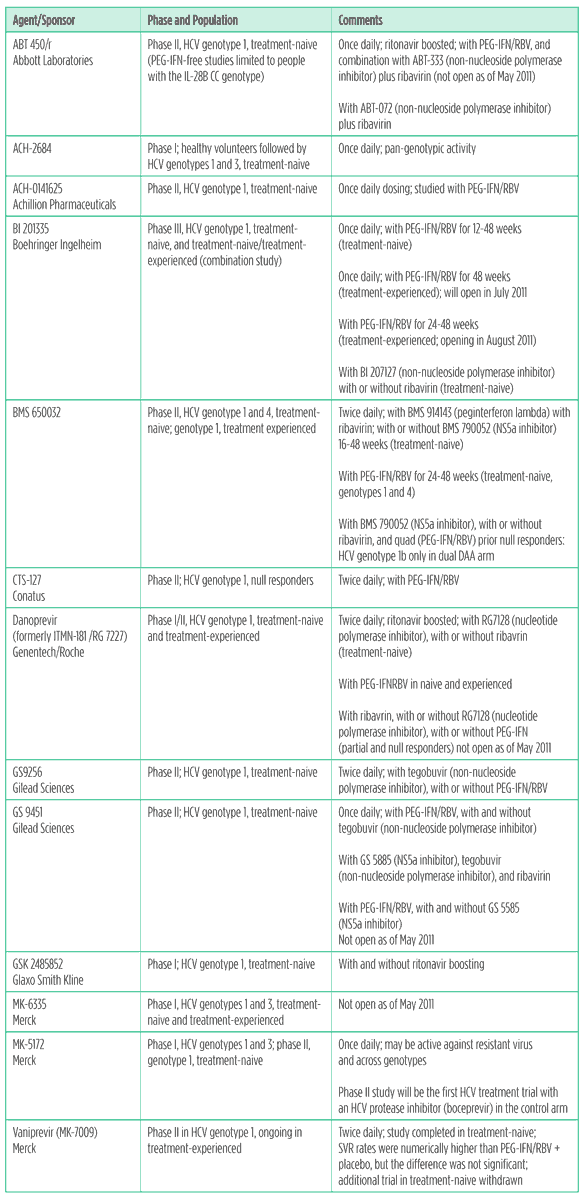
Boceprevir and telaprevir have the market to themselves for the next couple of years, but the next generation of HCV protease inhibitors is already nipping at their heels. These drugs offer advantages such as more convenient dosing, activity against other genotypes, and/or against protease resistant virus. Many are being studied in combination with other DAAs, with and without peginterferon, and with or without ribavirin.
Side effects include anemia, neutropenia, thrombocytopenia, photosensitivity, itching, rash, hemorrhoids, dysgeusia, headache, elevated alanine amino transferase (ALT) and bilirubin, jaundice, elevated uric acid and gout, dizziness, nausea, vomiting, and diarrhea.
Table 4. HCV protease inhibitors in development
NS5a inhibitors
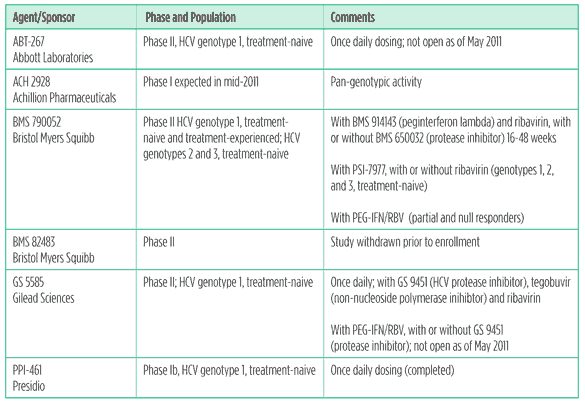
Although NS5a inhibitors are very potent, these drugs have a low resistance barrier, especially in HCV genotype 1a. Nonetheless, HCV has already been cured with an NS5a inhibitor combined with a protease inhibitor.This drug class is expected to become an important part of DAA regimens, since NS5a inhibitors may have pan-genotypic activity. So far, little is known about the side effect profile of NS5a inhibitors, since they have mainly been studied with peginterferon, ribavirin, and other DAAs; headache was reported in early studies.
Table 5. NS5a inhibitors in development
Non-nucleoside polymerase inhibitors
The hepatitis C virus offers more than one binding site for non-nucleoside polymerase inhibitors, so it may be possible to combine drugs from this class with one another, and with DAAs from other classes. Unfortunately, non-nucleoside polymerase inhibitors are active only against HCV genotype 1, and have a low resistance barrier. Side effects include headache, abdominal pain, nausea, fatigue, rash, and elevated bilirubin.
Table 6. Non-nucleoside polymerase inhibitors in development
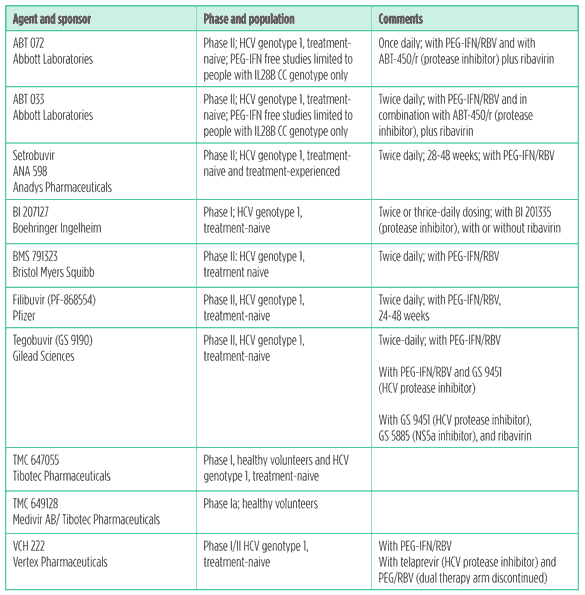
Nucleoside and nucleotide polymerase inhibitors
Although several candidates never made it out of phase II due to toxicity, the current nucleoside and nucleotide polymerase inhibitor candidates hold great promise. Early results from trials of Pharmasset’s PSI-7797 have generated hope that nucleotides may become the next backbone of HCV treatment – or the treatment itself.
Simplicity is king; nucleoside and nucleotide polymerase inhibitors may bypass current complexities of HCV treatment, such as IL28-B genotype, baseline viral load, race, HIV status, and HCV genotype. These drugs are not magic bullets, but nucleosides and nucleotides offer the potential to dramatically simplify and shorten HCV treatment, along with other desirable elements: a high resistance barrier, once-daily dosing, good tolerability, and pan-genotypic activity. Side effects include dizziness, fatigue, headache, and fever.
Table 7. Nucleoside and nucleotide polymerase inhibitors in development
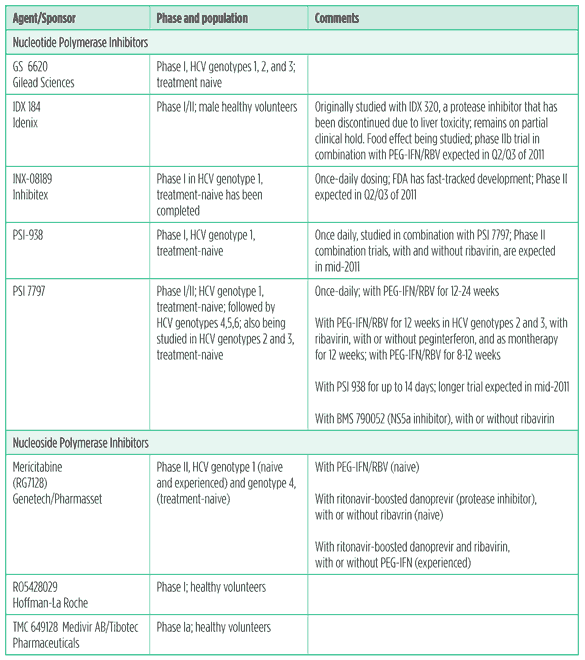
Host-targeting agents
Resistance to host-targeting agents (HTAs) is less likely to occur than DAA resistance, making these drugs an attractive addition to HCV treatment. There are different types of HTAs. Entry inhibitors work by blocking viral entry into host cells. Cyclophilin inhibitors work by binding to cellular proteins that regulate the immune system; some drugs in this class are immunosuppressants. Both Debio 025 and SCY-635 bind to host cell proteins that may facilitate HCV replication without immunosuppressive activity. Cyclophilin inhibitors may have pan-genotypic activity. Unfortunately, resistance to these drugs has been characterised. Side effects include muscle weakness, low platelets, headache, nausea and elevated bilirubin, which was reversible upon discontinuation.
Table 8. Host targeting agents in development
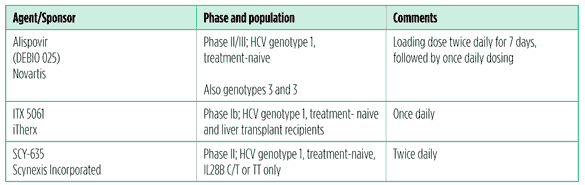
Novel interferons
If DAA combination therapy doesn’t pan out for everyone, new types and formulations of interferon will come in handy. These may be more convenient, or more tolerable. For example, peg lambda interferon was more effective at 12 weeks than peginterferon alfa 2a, as well as more tolerable. Flulike symptoms, anemia, neutropenia and thrombocytopenia were significantly lower among people who got peg lambda than peginterferon alfa, although the incidence of depression was similar.Transient elevations in direct bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were reported in the highest-dose peg lambda arm, but resolved when the dose was lowered or discontinued.9
Generic peginterferon could make HCV treatment accessible to millions of people who need it. There is at least one generic peginterferon (y-shaped peginterferon alfa 2a) in development, which hopefully will make HCV treatment accessible to the millions who need it and are unable to afford Merck’s Peg Intron or Roche’s Pegasys. In any case, patents in the United States and Europe will be expiring in 2016 (PegIntron) and 2017 (Pegasys).
Table 9. Novel interferons in development
Immunomodulators
Exploration of additional ways to stimulate the immune response against HCV continues, with a variety of different approaches, including monoclonal antibodies, therapeutic vaccines, and TLR-7 agonists.
Table 10. Immunomodulators in development
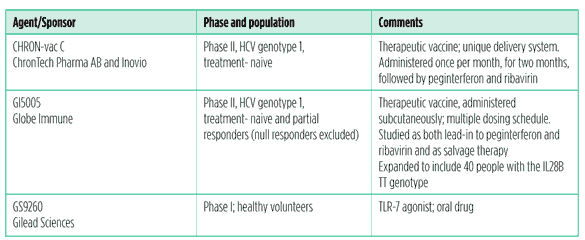
And more….
Other approaches to HCV treatment are being studied: Santaris Pharma’s injectable microRNA inhibitor, SPC3649 is entering phase II; it is being studied in treatment-naive people with HCV genotype 1.
Silymarin, the active ingredient in milk thistle, is being studied in acute viral hepatitis, as monotherapy and with peginterferon and ribavirin in treatment-experienced people.
Conclusion: getting ducks in a row
Investments in research and drug development have paid off for hepatitis C. We need a parallel investment in health care, to fully realise the benefits of therapeutic advances. HCV drug development is moving forward rapidly, but the capacity and resources to treat people with HCV have stalled. Millions of people are without access to HCV treatment; high drug prices and the global fiscal crisis make attainment of universal access a challenge. Access to drugs is not all that is required. HCV care and treatment must be offered with linkage to mental health care, case management services, peer support, addiction treatment, and harm reduction services.
Unfortunately, boceprevir and telaprevir were approved in the absence of treatment guidelines (aside from their prescribing information). Clinicians need information about how best to use these new drugs. Otherwise, therapeutic chaos may ensue, leading to treatment failure and drug resistance.
Our biggest challenge is not curing hepatitis C, it is getting health care systems ready for the people who will be using them, as we prepare people to deal with these fragmented systems. Hepatitis C is prevalent among poor, marginalised people. Many of them struggle with addiction, psychiatric disorders and medical comorbidities as well as socioeconomic challenges such as homelessness, unemployment, poverty and incarceration. Multidisciplinary HCV care and treatment, including peer support and education programmes, is effective, and these delivery systems need to be expanded. Developing and marketing new drugs will not cure people; good health care will.
References
- Lok A, Gardiner D, Lawitz E, et al. Quadruple therapy with BMS-790052, BMS-650032 and PEG-IFN/RBV for 24 weeks results in 100% SVR12 in HCV genotype 1 null responders. (Abstract 418) 46th Meeting of the European Association for the Study of the Liver. Berlin, Germany. March 30-April 3, 2011.
- Jacobson IM, McHutchison JG, Dusheiko G, et al ; ADVANCE Study Team. Telaprevir in combination with peginterferon alfa-2a and ribavirin in genotype 1 HCV treatment naive patients: final results of phase III ADVANCE study. (Abstract LB-2) 61st Annual Meeting of the American Association for the Study of Liver Diseases. Boston, Massachusetts. October 29-November 2, 2010.
- Poordad F, McCone J Jr, Bacon BR, et al; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1195-206.
- Sherman KE, Flamm SL, Afdhal NH, et al; ILLUMINATE Study Team. Telaprevir in combination with peginterferon alfa-2a and ribavirin for 24 or 48 weeks in treatment-naive genotype 1 HCV patients who achieved an extended rapid viral response; final results of phase III ILLUMINATE study. (Abstract LB-1) 61st Annual Meeting of the American Association for the Study of Liver Diseases. Boston,Massachusetts.October 29-November2,2010.
- Zeuzem S, Andreone P, Pol S, et al. REALIZE trial final results: telaprevir-based regimen for genotype 1 hepatitis C virus infection in patients with prior null response, partial response, or relapse to peginterferon/ribavirin (Opening and General Session 1) 46th Meeting of the European Association for the Study of the Liver. Berlin, Germany. March 30-April 3, 2011.
- Kasserra C, Hughes E, Treitel M, Gupta S, O?Mara E Clinical Pharmacology of BOC: Metabolism, Excretion, and Drug-Drug Interaction. (Abstract 118). 18th Conference on Retroviruses and Opportunistic Infections. Boston, Massachusett. February 27-March 2nd 2011.
- van Heeswijk R, Vandevoorde A, Boogaerts G, et al. Pharmacokinetic Interactions between ARV Agents and the Investigational HCV Protease Inhibitor TVR in Healthy Volunteers. (Abstract 119) 18th Conference on Retroviruses and Opportunistic Infections. Boston. February 27-March 2, 2011.
- Bacon BR, Gordon SC, Lawitz E, et al; HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic hepatitis C genotype 1 infection. N Engl J Med. 2011 Mar 31; 364 (13): 1207-17.
- Zeuzem S, Arora S, Bacon B, et al; on behalf of the EMERGE Study Group Pegylated Interferon-lambda (PegIFN-?) shows superior viral response with improved safety and tolerability versus PegIFN?-2a in HCV patients (G1/2/3/4): Emerge Phase IIB through week 12. (Abstract 1360) 46th Meeting of the European Association for the Study of the Liver. Berlin, Germany. March 30-April 3, 2011.
Additional resources
- http://www.clinicaltrials.gov
- http://www.hepatitisCadvocate.org
- http://www.hivandhepatitis.com
- http://www.natap.org

