Introduction and executive summary
14 July 2011. Related: Editorial, Pipeline report.
Polly Clayden and Mark Harrington
As this i-Base and TAG 2011 Pipeline Report makes clear, medically, the prospect for people with HIV, hepatitis C virus (HCV), and tuberculosis (TB) to live long and healthy lives – and in the cases of HCV and TB, to be cured rapidly with safe, effective, oral combination therapy – have never been better.
Politically and economically, the world’s activists and political leaders face a crisis in which the former must persuade the latter to redirect billions of dollars from unproductive wars into life-saving health research and access programmes, at home and internationally.
Here we will summarise the exciting progress that appears later in this report in great detail, and will look in greater depth at the last and the next decades of HIV treatment research.
Globally, 34 million people are living with HIV infection, an estimated 2 billion with latent Mycobacterium tuberculosis (TB) infection, and up to 130 million with chronic HCV infection.
At least 1.8 million people died from AIDS in 2009, one quarter of them from TB, which on its own killed 1.7 million people. There is neither global nor national surveillance for HCV-related illness and death, but more than 300,000 people die from HCV complications each year, and HCV mortality will continue to increase in the coming decade.
HIV infection can be controlled with lifelong triple-combination antiretroviral therapy (ART). Latent TB infection can be treated with six to nine months of isoniazid (INH) or 12 weeks of once-weekly rifapentine and INH. Active TB disease, if drug-susceptible, can be cured in 95% of cases with four drugs in six months, while drug-resistant forms of the disease can be cured up to 70% of the time if multidrug-resistant, or just 30% if extensively drug-resistant, with unpleasant combinations that can take up to two years to work, if they work at all. HCV is now curable in up to 75% of infected people with genotype 1 (predominant in major pharmaceutical markets) who have access to – and can tolerate – today’s standard of care: triple therapy with pegylated interferon, ribavirin, and an HCV protease inhibitor.
As the writers of this report reveal, the prospects for dramatic – indeed in some cases revolutionary – changes in prevention and treatment for the three diseases in the next decade are amazingly good. Decades of high-quality research, increased investment, and growing and targeted community-based activism have set the scene for the possibility – for the first time since HIV/AIDS emerged in 1981 – to make dramatic reductions in new HIV infections worldwide, while saving the lives of as many of the 34 million currently infected who can access therapy. Treatment is continually improving, with modern combinations dramatically less toxic, more tolerable, and easier to take than the first-generation ART combinations of the 1990s.
Simon Collins covers developments in the innovator antiretroviral (ARV) treatment pipeline, while Polly Clayden addresses the persistent, and as-yet-unfulfilled, needs of the substantial global population of infants, babies, children, and adolescents with HIV for appropriate and easy-to-use formulations of the best ARV drugs.
Contrary to the predictions of obstinate pessimists who constantly bemoan the imminent emptying of the ARV pipeline, Simon Collins demonstrates that the 2011 HIV treatment pipeline is robust indeed, with twelve agents and fixed-dose combinations (FDCs) in phases II or III, still more in phase I, and two new drugs or formulations already approved in the last year – the NNRTIs rilpivirine (Edurant) from Tibotec/J&J (although when this will be preferred in treatment-naive patients is unclear), and Boehringer Ingelheim’s extended-release formulation of nevirapine, Viramune XR (just in time for the patent expiry on the original). Two new integrase inhibitors – elvitegravir and dolutegravir – are in late stages of development, both formulated in novel FDCs. This year’s pipeline is at least as full as that of any year documented by TAG in our annual ARV pipelines since 2003.
Polly Clayden provides an intriguing and expanded summary of the emerging field of point-of-care diagnostics research to make HIV-RNA (viral load) and CD4 cell-count testing available in decentralised settings where most people will receive their HIV treatment and care over the coming decade.
Although, as Jonathan Berger explains, the empty pipeline pessimists may be right for the global majority of people with HIV. For most people in developing countries, access to new drugs is hostage to a number of non-medical factors, particularly those related to intellectual property. In his chapter, Berger examines the global context that affects domestic patent laws. He also provides valuable insight into the licensing policies of companies with ARVs in the pipeline.
Also for the first time, researchers and activists are seriously pooling forces to mount a campaign to accelerate research to actually cure HIV infection. Richard Jefferys provides a clear summation of the state of the art of HIV cure-related research. Jefferys also describes an encouraging duo of promising results from ART-related prevention strategies: the tenofovir gel?containing HIV microbicide used in the CAPRISA 004 study and the oral Truvada (emtricitabine/tenofovir) preexposure prophylaxis (PrEP) pill used in the iPrEx study in gay and transgender people. These results, taken together with the stunning 96% reduction in HIV acquisition among seronegative partners whose HIV positive partner received immediate ART at CD4 counts between 350-550/mm3 in HPTN 052, constitute a seismic shift in biomedical HIV-prevention.
We eagerly await the results of the ongoing Strategic Timing of Antiretroviral Therapy (START) trial to further document the size and quality of the benefits of earlier ART initiation among treatment-naive persons entering with at least 500 CD4 cells/mm3, which will complement those of HPTN 052 and clarify whether ART should actually be indicated at the time of HIV diagnosis for most, if not all, infected persons.
Jefferys continues his long-standing and detailed assessment of the HIV preventive and therapeutic vaccine pipelines along with those for immune-based, cell-based, and gene therapy approaches to HIV treatment and functional cure.
In the case of HCV – as Tracy Swan’s overview demonstrates – several generations of new direct-acting antivirals (DAAs) are in the pipeline, holding out the promise that it may be possible to cure people with oral drugs in the future. The HCV pipeline is robust. Currently, 14 HCV protease inhibitors (not including the just-approved boceprevir and telaprevir), 6 NS5a inhibitors, 10 non-nucleoside polymerase inhibitors, 8 nucleoside or nucleotide polymerase inhibitors, 3 host-targeting agents, 4 novel interferons, 3 immunomodulators, a microRNA inhibitor, and an extract of milk thistle are in development.
If the promise of all-oral DAA cures is realised, the potential to roll out HCV treatment globally would then become dramatically easier, and hundreds of millions of lives could be saved. But most people with hepatitis C will not be cured – or even treated. The drugs are simply too expensive. New HCV treatments must be accessible to those who need them.
Swan warns that neither the health care system nor the provider community is ready to administer new and complex HCV regimens to an onslaught of newly diagnosed patients. Major adjustments will be needed to ensure that people with HCV – who often may need mental health care, addiction treatment, and HIV care and treatment – can be treated with dignity. Swan recommends the immediate establishment of a standing, multidisciplinary federal HCV treatment-guidelines panel – modeled in part after the very successful DHHS Antiretroviral Therapy Guidelines panels for adults, adolescents, and children – to review new data as they emerge, and to promulgate a coherent and up-to-date standard of care for all individuals with HCV.
Swan is critical of the pharmaceutical industry’s failure to provide early-access trials and programmes for people at risk for progression to end-stage liver disease. Preapproval access to new HCV drugs will save lives, and inform clinical practice in patients with urgent need. Swan is also dismayed that HCV drugs can come to market without information on how to safely and effectively use them during HIV treatment, with methadone, or in combination with other commonly used medications – or that coadministration may not be possible.
With TB, one of humanity’s oldest and most stubborn pathogens, recent developments are encouraging but not yet revolutionary. A new, rapid, accurate, and sensitive TB test is now being rolled out worldwide which can diagnose the disease in two hours rather than two months. It works especially well in the forms of TB common among people with HIV infection, and it also detects drug-resistant TB. Proper deployment of this test could accelerate the identification and proper treatment of millions of people with TB disease. Its drawbacks include its price and requirement for electricity, and the lack (to date) of data to guide its use in children.
No new treatment has been approved to treat TB since the 1960s. For the first time in four decades, two new drugs from two new classes (TMC207 and OPC-67683) are likely to be submitted to regulatory authorities for approval in the coming year to treat multidrug-resistant forms of TB. Again, the potential gains in lives saved amount to millions.
Claire Wingfield and Richard Jefferys provide an encouraging assessment of recent developments in the slowly reviving TB vaccine research field, while Wingfield herself documents the results of the past decade of increasing investment in TB treatment, which is finally beginning to yield promising candidates. Javid Syed notes with dismay the sudden lull in development of new TB diagnostic tests after a brief spurt in the past four years – with the elusive TB point-of-care (POC) test still a distant aspiration – while the rollout of Cepheid’s Xpert MTB/RIF TB and drug-resistance rapid molecular test has the promise of radically accelerating TB diagnosis and proper treatment initiation among persons with HIV-associated or drug-resistant TB. The next year will see extensive implementation science related to the rollout of Xpert MTB/RIF, while basic scientists and industry technicians continue their arduous, grossly underfunded search for a potential biomarker that could be used in a TB POC test.
Over the past decade, the public, private, and philanthropic sectors have invested tens of billions of dollars in HIV prevention and treatment research, hundreds of millions in HCV, and far less in TB, despite its prevalence, persistence, and toll on human lives. Clearly diseases that affect both rich and developing countries attract far more research investment than those predominantly confined to the latter. Political leaders and treatment activists alike have the obligation to redouble their efforts in the coming decade to accelerate research that could end the pandemics of HIV, HCV, and TB. In the meantime, political leaders must redirect resources from wars and serial bank bailouts toward meeting the health needs of their own people and everyone else around the world.
A golden decade of antiretroviral drug development
We believe it is worth exploring the past decade of ARV drug development for several reasons, including (1) to evaluate claims that the HIV drug pipeline is drying up; (2) to determine the success rate for ARV drug candidates entering phases II?III in order to assess the likelihood that current candidates will progress toward approval; (3) to examine the rapidity with which new drugs and combinations enter practice in one industrialised country, the United States; and (4) to discuss the relative potential of investments in studying lower doses of existing drugs versus expanding investment in new drugs and combinations.
The global ARV treatment market is estimated at $13 billion in market volume this year (Market Research News 2011), with most of the profits made in industrialised countries, while most of the people in need of treatment live in developing ones.
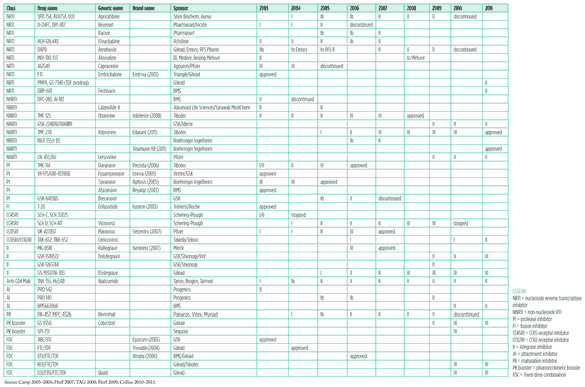
The past decade has indeed been a golden age of ARV drug development. Of the 29 new chemical entities approved by the U.S. Food and Drug Administration (FDA) to treat HIV infection since 1986, almost half (14/29) were approved in the years since 2003 (FDA 2011a). Thirty-four drugs and FDCs are FDA-approved for sale in the United States; a further 131 drugs and FDCs (including adult and paediatric formulations) are tentatively approved under the FDA’s generic registration programme to facilitate global access through programmes such as the President’s Emergency Programme for AIDS Relief (PEPFAR) (FDA 2011b). Please see the data series from the TAG ARV pipelines dating from 2003 to the present (Table 1, pp. 6-7).
The success rate for new ARV drugs and FDCs that have entered phase II or further studies since 2003 is an astonishing 30.4% (14/46), with approval believed to be likely in the coming year for the integrase inhibitor elvitegravir, the pharmacokinetic booster cobicistat, and the two FDCs rilpivirine/FTC/TDF and elvitegravir/cobicistat/FTC/ TDF (?Quad?) – which would bring the success rate to 39.1% (18/46).
So much for those who say investing in HIV treatment is a bad bet.
Table 1. HIV treatment pipeline 2003?2011
Click on table image below to see larger version
Sources: Camp 2003?2006; Huff 2007; TAG 2008; Huff 2009; Collins 2010?2011.
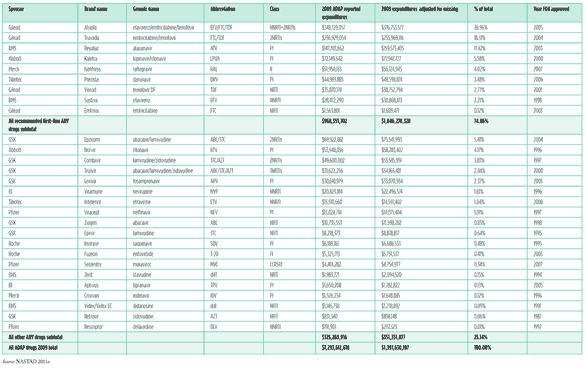
Table 2. US ADAP antiretroviral market share by drug in 2009
Click on table image below to see larger version
Source: NASTAD 2011a
Rapid implementation and uptake of new therapies provides rapid return on investment
Another remarkable feature of the HIV treatment landscape is the rapidity with which new drugs and combinations are incorporated into the standards of HIV care in developed countries. In the United States, this process has been facilitated since the late 1990s by the establishment of the standing Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents and the Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children (http://aidsinfo.nih.gov/Guidelines/Default.aspx).
The Adult and Adolescent Guidelines panel meets monthly by teleconference, once yearly in person (usually at the annual retrovirus conference CROI), and issues updated online treatment recommendations at least annually, with changes highlighted in yellow to make navigating the ever-changing treatment landscape easier. A review of data generously provided by the U.S. National Association of State and Territorial AIDS Directors (NASTAD) demonstrates the astonishing fidelity of US AIDS Drug Assistance Programme (ADAP) prescribing practices in 2009 to the most recent iteration of the US HIV treatment guidelines (Table 2, pp. 8-9).
Among the many striking features of the 2009 ADAP reported data on ARV usage are (1) 75% of sales were for drugs recommended as preferred first-line ART regimens in the federal guidelines (Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents 2009); (2) of the nine drugs and FDCs included among the US first-line recommendations, eight of nine were approved by the FDA in the past decade; just one (efavirenz) was approved in the 1990s; (3) two drugs approved in 2006 and 2007 – darunavir and raltegravir, respectively – were approved by the FDA for a first-line indication less than two years after initial recommendation in salvage patients; (4) both of those drugs soon after were included by the US guidelines panel among the preferred recommended regimens for antiretroviral-naive patients; and (5) prescribing practice rapidly evolved to incorporate the newest data on the newest drugs.
These data demonstrate the effective interaction of research, regulation, normative guidelines, practice, and implementation in the United States, despite its highly fragmented health care system and the fact that those receiving treatment through ADAP are, by definition, not rich. However, the fact that over 8,000 people are currently on waiting lists to receive treatment through ADAP reveals that not all is rosy in the United States (NASTAD 2011b).
In any case, it is clear that HIV therapeutics has room for considerable improvement, and that improvements will be rapidly diffused and their investors will enjoy a substantial return on their investment. We urge the World Health Organization (WHO) as well as national guidelines-defining groups in countries affected by HIV to urgently implement such forward-looking practices to ensure that people living with HIV everywhere have access to the best possible treatment options.
Less of the old – or more of the new?
There is a fine balance between innovation and retrofitting. There is a lot of talk underway about ?dose reduction? or ?optimization? strategies to allow the cheaper and potentially less toxic use of certain existing and possible new drugs, as summarised in Table 3.
Table 3. Proposed potential ARV dose reduction studies and savings
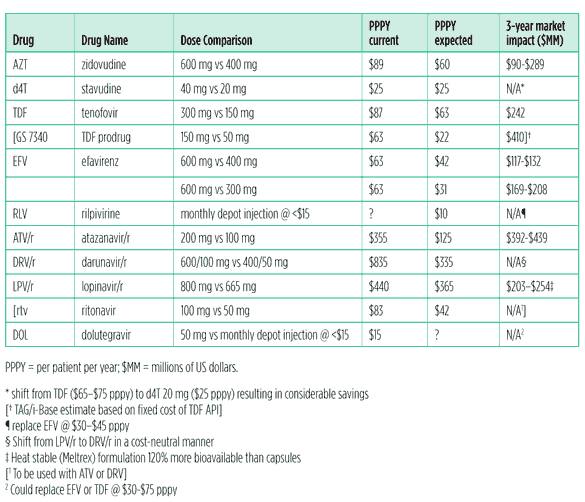
Source: Adapted from Conference on ARV Dose Optimization, Clinton Health Access Initiative, John Hopkins University, School of Medicine. Meeting Summary; Prioritised Project Portfolio by ARV. Unpublished manuscript. Conference on Antiretroviral Dose Optimization, Alexandria, VA, 7-10 June 2011.
[i-Base/TAG interpolations enclosed within brackets]
A minimal requirement for a reduced-dose ARV drug to be considered for use in global guidelines directed particularly at resource-limited settings would include proof of efficacy and safety. ARV activity needs to be comparable to currently recommended drugs and combinations. The acceptable margins of reduced activity have yet to be defined. Safety for long-term use in places where laboratory capacity is nonexistent and clinical monitoring is minimal at best, will also have to be proven. Particularly where patients and their families/support groups will be required to provide pharmacovigilance.
Some dose-reduction strategies might well succeed and are justified by the potential to free up substantial sums to be spent on expanding the number of people able to receive treatment. However, we have serious concerns about the proposed dose-reduction study of stavudine (d4T), a drug that, though cheap to manufacture, is on its way out everywhere in the developed world and in many places in the developing one. While the proposed 20mg stavudine dose might be acceptable in a short-term 48- or even 96- week virologic endpoint study, many of its most serious side effects (such as peripheral neuropathy and lipoatrophy) would not necessarily emerge until after such a study was completed.
For these reasons, we do not believe the stavudine dose-reduction study should proceed. Activist pressure and research needs to focus on increasing access to safer cost-saving alternatives to stavudine.
Even greater savings could be achieved if the tenofovir prodrug GS 7340 could actually be given at 50 mg daily or less – studies are still underway to determine its dose moving forward. Since the current global best-price for TDF is about $87 (MSF 2011), bringing the volume of active pharmaceutical ingredient for tenofovir down by 5/6 might allow a lowest global price of $22 or less – lower than the putative 20 mg stavudine price of $25.
Other drugs in late-stage development such as the integrase inhibitor dolutegravir (50 mg once daily) also offer potential savings on manufacturing.
Developers are also considering novel manufacturing and delivery techniques such as nanoparticles – discussed further by Simon Collins below – and long-acting slow-release injectables, which could potentially be given monthly or even quarterly. These compounds will never develop without financial support that recognises the potential impact they could have on global health.
Therefore, we believe a proper balance needs to be struck between repurposing old or existing drugs with a search for the lowest safe and effective dose, and investing in innovative discovery and delivery systems which could potentially lead to much better outcomes for all.
Conclusion and recommendations
Just 40%, or 6 million, of the estimated 16 million HIV-positive people in need of immediate treatment are currently receiving it. Implementers of HIV treatment programmes in developing countries are facing awkward decisions about the speed at which they will continue to enroll new patients into HIV treatment programmes while maintaining recently enrolled ones. In addition to raising the enrollment bar to admit people with higher CD4 cell counts (up to 350 cells/mm3 and in some cases higher), switching from stavudine-based to tenofovir-based first-line regimens, strengthening the quality of patient and laboratory monitoring, and expanding prevention and testing programmes. All in the context of a gaping abyss of global leadership, flagging political commitment, and uncertain prospects for economic revival.
Activists, scientists, implementers, and political leaders are obliged to exert their utmost efforts to accelerate scientific progress and to save as many lives as possible in spite of the challenges we face. In this concluding section of the introduction to the 2011 Pipeline Report, we highlight some of the most pressing priorities for research, access, and activism for HIV, HCV, and TB, emphasising, when possible, opportunities for cross-cutting integration of efforts.
HIV
Despite the unprecedented progress in both research and access over the past decade, many unmet medical and public-health needs remain for future HIV treatments and programmes.
- Point-of-care diagnostics. The promise of universal access cannot be met without cheap, accurate, point-of-care diagnostic tests to diagnose HIV, stage patients for risk of opportunistic complications, and monitor response to therapy. Thus, intensified research efforts are required to accelerate the development, uptake, and rollout of point-of-care HIV diagnostics, and viral-load and CD4 tests.
- Simple, safe, and durable daily FDCs. Unmet medical needs in the HIV therapy space are many. A cheap, daily (or less often) FDC that is super-potent, safe, tolerable, nontoxic, and with a high barrier to resistance – and that can be used in children, pregnant women, and those with TB – would make it possible to keep most people on first-line therapy for life (or until a cure is discovered and made readily available worldwide).
- New anchor drugs. For adults globally, a new NNRTI (or a super-low molecular weight protease or integrase inhibitor) that spares the CNS, kidneys, lipids, and liver from currently common toxicities could be a great advance.
- New backbone drugs. Something cheaper than tenofovir (TDF) would be good; a TDF prodrug such as GS 7340 could fit in nicely here. Novel nucleoside backbones which do not use tenofovir or AZT could be helpful, both for toxicity reduction and for greater strategic options in second-line therapy and beyond.
- New paediatric drugs, formulations, and FDCs. Accelerated development of new drugs in appropriate paediatric formulations or FDCs for children of all age levels would help to close the gap between the quality and modernity of adult and paediatric HIV treatment.
- Integration of ART-based prevention and treatment. With the recent breakthroughs on the microbicide, PrEP, and serodiscordant couples/early ART-initiation fronts, programme implementers finally have a chance to end the sterile debates and bureaucratic fragmentation of HIV programmes that fail to address testing, prevention, treatment, and care in an integrated and community-driven manner.
- Complete the START trial. Given the results of HPTN 052, it is essential to complete the START trial to provide more quantitative and qualitative data on the risks and benefits of much earlier initiation of ART. And to answer, in sub-studies, questions on the impact of HIV on central nervous system (CNS) and bone health in early infection.
- Invest in treatment for people currently resistant to existing drugs – for whom tolerability may be different from that of treatment-naive people – including the strategic development of new FDA-supported initiatives to study two or more compounds active against MDR-HIV together in joint fast-access protocols.
- Invest in and accelerate research toward a functional and a sterilising cure for HIV.
- Continue to plug away at discovering and developing a truly effective preventive vaccine.
- In the meantime, further validate ARV-based microbicides and PrEP, and figure out how to use them effectively in the real world.
- The WHO should move much more rapidly to update its HIV treatment and prevention guidelines to respond to newly emerging data.
HCV
- Establish national-level HCV treatment guidelines panels to regularly update the standard of care for HCV treatment based on the latest data. Prepare health systems, providers, and people with HCV for the rapidly coming day when HCV will be curable with all-oral DAAs.
- Plan for global uptake and access to all-oral DAA cures once they are available.
TB
- Substantially intensify investment in new TB diagnostics, drugs, vaccines, and operational research to support scale-up of new technologies. Accelerate discovery and development of a true TB POC test.
- Encourage the entry of new sponsors in TB drug and vaccine development.
- Prioritise development of paediatric formulations and FDCs for current and new TB regimens.
- Study the safety of TB drugs in pregnant women.
- Cross-link existing research infrastructure and develop new site and network capacity to more rapidly evaluate new TB treatment regimens and preventive vaccines.
References
Camp R. Antiretrovirals Pipeline Report. New York: Treatment Action Group, 2003. Camp R. Antiretrovirals Pipeline Report, 2004. New York: Treatment Action Group, February 2004.
Camp R. Antiretroviral Treatment Pipeline 2005. What’s in the Pipeline: New HIV Drugs, Vaccines, Microbicides, HCV and TB Treatments in Clinical Trials. New York: Treatment Action Group, July 2005.
Camp R. Antiretroviral Pipeline 2006. What’s in the Pipeline: New HIV Drugs, Vaccines, Microbicides, HCV and TB Therapies in Clinical Trials. New York: Treatment Action Group, August 2006.
Clinton Health Access Initiative, John Hopkins University, School of Medicine. Meeting Summary. Unpublished manuscript. Conference on Antiretroviral Dose Optimization, Alexandria, VA, June 7?10, 2011.
Collins S. The Antiretroviral Pipeline. TAG 2010 Pipeline Report: HIV, Tuberculosis, and Viral Hepatitis: Drugs, Diagnostics, Vaccines, Immune-Based Therapies, and Preventive Technologies in Development (Second Edition). New York: I-Base and Treatment Action Group, September 2010.
Collins S. Antiretroviral Pipeline 2011. 2011 Pipeline Report: HIV, Hepatitis C Virus (HCV), and Tuberculosis Drugs, Diagnostics, Vaccines, and Preventive Technologies in Development. July 2011.
Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 1 December 2009. Accessed 23 June 2011 at
http://aidsinfo.nih.gov/ contentfiles/AdultandAdolescentGL001419.pdf
Food and Drug Administration (FDA 2011a). Antiretroviral drugs used in the treatment of HIV infection. Accessed 22 June 2011 at
http://www.fda.gov/ForConsumers/byAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118915.htm
Food and Drug Administration (FDA 2011b). President’s Emergency Plan for AIDS Relief: Approved and Tentatively Approved Antiretrovirals in Association with the President’s Emergency Plan. Accessed 22 June 2011 at
http://www.fda.gov/InternationalPrograms/FDABeyondOurBordersForeignOffices/AsiaandAfrica/ucm119231.htm
Hill A, Ananworanich J, Calmy A. Dose optimisation: A strategy to improve tolerability and lower antiretroviral drug prices in low and middle income countries. Open Infect Dis J. 2010;4:85?91. Accessed 22 June 2011 at
http://www.benthamscience.com/open/toidj/ openaccess2.htm
Huff B. The Antiretroviral Drug Pipeline. 2008 Pipeline Report: HIV, Tuberculosis, Hepatitis B, and Hepatitis C: Drugs, Diagnostics, Vaccines, and Microbicides in Development. New York: Treatment Action Group, July 2008.
Huff B. Antiretroviral Drug Development in 2009. TAG 2009 Pipeline Report: HIV Tuberculosis Viral Hepatitis Drugs, Diagnostics, Vaccines, and Microbicides in Development. New York: Treatment Action Group, July 2009.
Market Research News. The HIV/AIDS Market Outlook to 2015: Competitive landscape, market size, pipeline analysis and growth opportunities. 14 March 2011. Accessed 22 June 2011 at
http://www.salisonline.org/market-research/the-hivaids-market-outlook-to2015-competitive-landscape-market-size-pipeline-analysis-and-growth-opportunities/
Medecins sans Frontiers. MSF Campaign for Access to Essential Medicines. Untangling the web of antiretroviral price reductions. 2011 edition. Accessed 22 June 2011 at
http://utw.msfaccess.org
National Association of State and Territorial AIDS Directors [NASTAD 2011a]. Raw data used in Table 2, US ADAP Antiretroviral Market Share by Drug in 2009.
National Association of State and Territorial AIDS Directors [NASTAD 2011b]. ADAP Watch – June 17, 2011. 17 June 2011. Accessed 22 June 2011 at
http://www.nastad.org/infocus/infocusresults.aspx
Treatment Action Group. The 2007 Pipeline Report: Experimental Treatments and Preventive Therapies for HIV, Hepatitis C, and Tuberculosis. New York: Treatment Action Group, July 2007.