Frailty in HIV care and the importance of lifestyle changes
15 November 2019. Related: Conference reports, Ageing and life expectancy, HIV and Ageing 2019.
 Simon Collins, HIV i-Base
Simon Collins, HIV i-Base
In the first plenary talk at the workshop, Linda Fried from the Mailman School of Public Health, Columbia University, presented an overview of approaches to frailty over the last three decades which has developed as a new discipline. [1]
Fried is a leading researcher in this field who helped define frailty as a syndrome that is independently predictive for serious comorbidities and mortality than just the factors that contribute to its definition.
Starting in the 1980s, Fried outlined that the initial idea of frailty was used interchangeably with disability in connection with comorbidity and ageing. However, within a decade, six component parts were better defined by geriatricians.
- Declines in lean body mass, strength.
- Weight loss.
- Loss of endurance.
- Slow walking.
- Relative inactivity.
- Decreased balance and morbidity.
Many of these factors were related in a cycle of frailty involving poor nutrition, loss of muscle mass, reduced physical activity, metabolic rate and walking speed. See Figure 1.
Figure 1. Cycle of frailty, Fried et al, 1998.
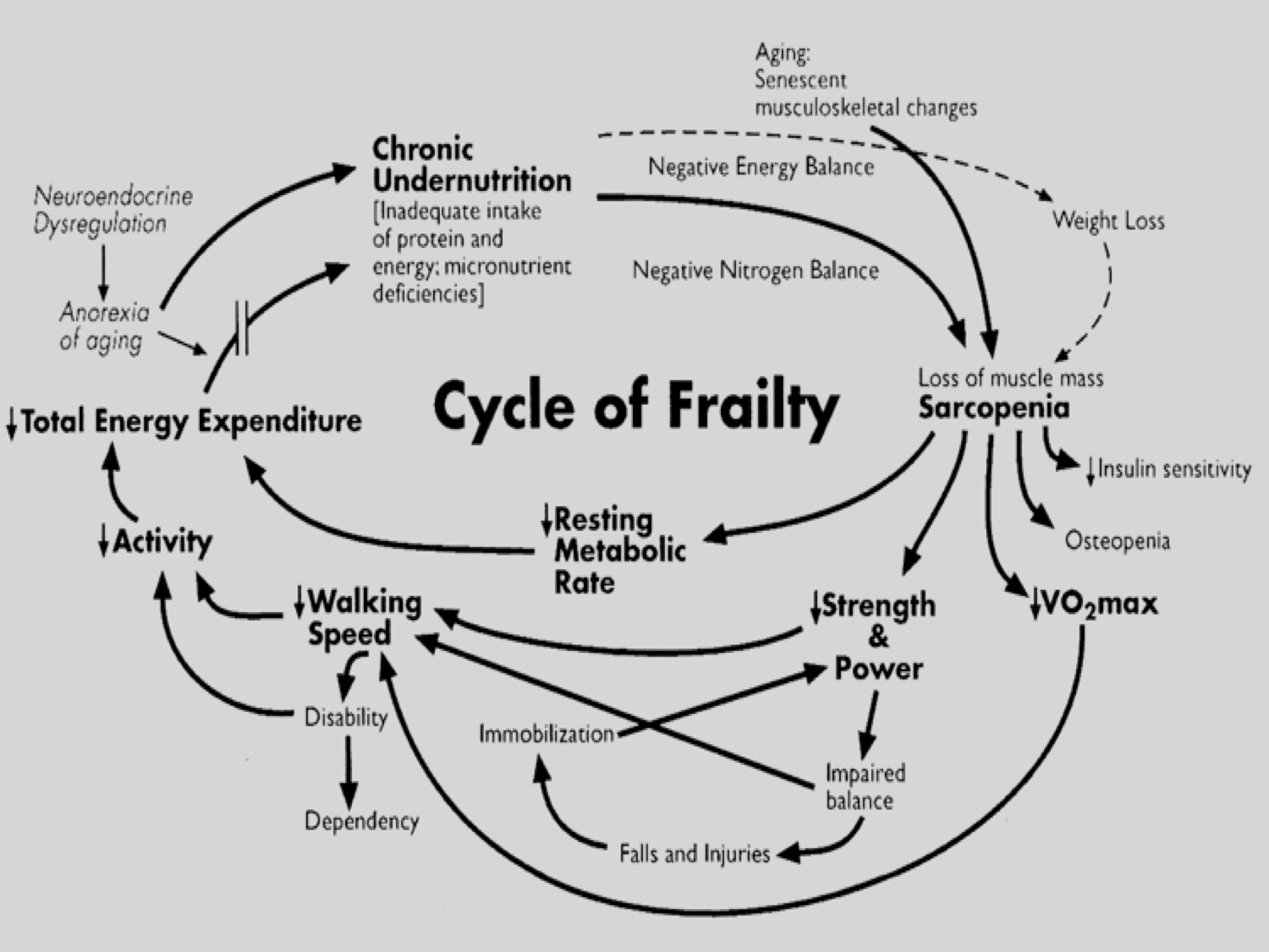
Each stage of the cycle was related and any point could start or accelerate progression to a frail state. For example, sarcopenia predicted lower strength, which would be further weakened by reduced exercise (affecting the resting metabolic rate,) which in turn slowed walking, which then limits further exercise. Often, a subset of adults with low activity who were not regulating their diet, become chronically undernourished, which then worsened sarcopenia and led to further weight loss etc.
The next decade saw the links between frailty factors become recognised as a geriatric syndrome, although there was still little explanation for how and why these component factors were related or for explaining differences between other patients who were ageing without frailty.
More recently, perhaps since 2015, frailty has been characterised as a medical syndrome linked to a distinct underlying pathology that is underpinned by other diseases with shared biologic pathways driven by ageing.
So frailty is now commonly contrasted as the inverse of resilience.
The new model developed by Fried and colleagues is focused on this cycle of dysregulation which can start at any point. Working with Russ Tracey and colleagues in a cardiovascular and frailty study helped define a frailty phenotype made up of shrinking, weakness, slowness, poor endurance and low activity, see Table 1. [2]
Table 1: Phenotype of frailty in CHS study, 2001 [2]
| Characteristic | Definition |
| Shrinking | Unintentional weight loss >10 lbs, past year; F/U: ≥ 5% weight loss over 1 year |
| Weakness | Grip strength: lowest 20% |
| Slowness | Walking time: lowest 20% |
| Poor endurance | Exhaustion (self report) |
| Low activity | Kcal/week: lowest 20% |
The presence of three or more of these factors were used to define frailty, with one or two factors defining an intermediate stage of prefrailty and no factors being non-frail.
Using this definition, a 2015 study of people in the US older than 65 years categorised 15% as being frail and 45% pre-frail. Importantly, not everyone who was frail had one or multiple chronic diseases and not everyone who was frail was disabled – but there was a significant overlap. The prevalence of frailty also increased geometrically with age, from 9% in those aged 65-69 to 38% in those aged 90 or above. [3]
Studies on cardiovascular disease and women’s ageing were used to validate frailty phenotype by showing the association with very high risk of serious outcomes. [2, 4]
For example, only 81% of participants diagnosed as frail at baseline were alive three years later in unadjusted analysis – but adjusting for 78 related factors still showed independent high risk of serious outcomes including mortality, disability, falls, hospitalisation, poor post-surgery outcomes and slow recovery from illness.
In an earlier study, 20/78 of these factors were independently and jointly predictive of frailty. [5]
This meant that ten years ago, researchers had validated a clinical presentation that increased with age and that had demographic differences: in the US, there are higher risks for women compared to men and for African Americans compared to Caucasian race. These data also supported a natural history where frailty had a very high risk for 6-month mortality and pre-frailty had a very high risk of becoming frail.
Over the last ten years specific biomarkers including dysregulation of inflammatory, endocrine and immune activation pathways have broadened the molecular and genetic factors of frailty.
Many of these studies showed similar characteristics between frail and non-frail participants at baseline, but then showed significantly different responses after exposure to stress tests.
For example, glucose and insulin dynamics were significantly increased (approximately twice as high and with at least three times as long for recovery) in a study of non-diabetic women (age 84 to 93) in response to glucose tolerance test. [6]
In a similar population, after a 30 second calf exercise, frail women had 41% slower and prefrail women 15% slower phosphocreatinine recovery, compared to non-frail participants. [7]
The implications of these dysregulations of energy homeostasis and longer recovery time sets up a biological vulnerability that can affect any component in the frailty phenotype. After adjusting for age, race, education, and number of chronic diseases, the risk of frailty is increased in a greater than linear way as an increasing number of physiologic systems dysregulated, see Table 2. [8]
Table 2: Odds Ratio (OR) for frailty based on number of dysregulated body systems
| Number of dysregulated systems | OR for frail vs non-frail | p-value |
| 0 | 1 | |
| 1-2 | 4.8 | <0.05 |
| 3-4 | 11.0 | <0.01 |
| 5+ | 26.0 | <0.01 |
Management of frailty
The compelling picture, where frailty drives a difference between ageing well and ageing with a higher risk of mortality, highlights the importance of lifestyle changes – given that no direct treatments are available for frailty itself.
The benefit of lifestyle interventions (diet, exercise, mobility etc) can help many of the individual factors in the frailty syndrome. The discussion of the evidence of each intervention showed the potential to reverse frailty. For example, even in older age, moving from a sedentary to more active lifestyle has been shown to reverse frailty factors and reduce frailty incidence. [9]
comment
As the HIV population steadily ages, a focus on frailty should help direct services to support HIV positive people at greatest risk of associated complications.
In the same month (and as further reading) the Lancet published two excellent overview articles on frailty including on the implications for clinical practice. [10, 11]
This later article includes an excellent practical review of exercise approaches to reduce the risk of sarcopenia. [12]
References
- Fried LP. 10 Years of aging research: frailty and aging. Plenary talk. 10th International Workshop on HIV and Ageing, 10-11 September 2019, New York.
- Fried LP et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146-56.
https://www.ncbi.nlm.nih.gov/pubmed/11253156 - Bandeen Roche K et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015 Nov; 70(11): 1427–1434. doi: 10.1093/gerona/glv133.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4723664 - Bandeen-Roche K et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006 Mar;61(3):262-6.
https://www.ncbi.nlm.nih.gov/pubmed/16567375 - Fried LP et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998 Feb 25;279(8):585-92.
https://www.ncbi.nlm.nih.gov/pubmed/9486752 - Kalyani RR et al. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci . 2012;67:1300–1306. doi: 10.1093/gerona/glr141.
https://www.ncbi.nlm.nih.gov/pubmed/21873592 - Varadhan R et al. Relationship of physical frailty to phosphocreatine recovery in muscle after mild exercise stress in the oldest-old women. J Frailty Aging 2019;8(4)162-168.
http://dx.doi.org/10.14283/jfa.2019.21 - Fried LP et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009 Oct; 64(10):1049-57. doi: 10.1093/gerona/glp076.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2737590 - Cesari M et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015, Feb;70(2):216-22. doi: 10.1093/gerona/glu099.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4311184 - Hoogendijk EO et al. Frailty: implications for clinical practice and public health. The Lancet. 394 (10206); 1365-1375. (12 October 2019).
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31786-6/fulltext - Dent E et al. Management of frailty: opportunities, challenges, and future directions. The Lancet. 394 (10206); 1376-1386. (12 October 2019).
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31785-4/fulltext - Lucas EJ. Unseen cost of weight loss and aging: tackling sarcopenia. Medscape. (16 October 2024).
https://www.medscape.com/viewarticle/unseen-cost-weight-loss-and-aging-tackling-sarcopenia-2024a1000inn

