Tecovirimat news continues: Japan approval includes mpox indication
2 January 2025. Related: mpox.
Simon Collins, HIV i-Base
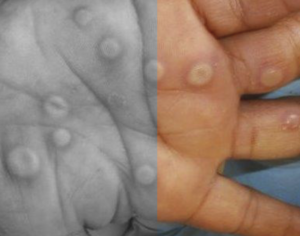 On 2 January 2025, Siga Technologies announced that tecovirimat had been approved in Japan with a broad indication to treat orthopoxviruses including smallpox, mpox and cowpox. [1]
On 2 January 2025, Siga Technologies announced that tecovirimat had been approved in Japan with a broad indication to treat orthopoxviruses including smallpox, mpox and cowpox. [1]
This news was unexpected given two large randomised mpox studies very recently reported no clinical benefits against mpox, with both studies being stopped early. [2]
When the first of these studies was stopped in October 2024, Siga claimed that data might still support potential benefits, even though this was denied by the NIH. [3]
comment
As tecovirimat was licensed for mpox in the UK in 2022 and in the EU in 2023, with a caution about very limited data, the Japanese decision might just be late and might be reevaluated now. If this is the case then SIGA would be opportunistically issuing misleading information.
Given the changing recommendations over the use of tecovirimat in the management of mpox and the lack of clearly effective treatments, these differences need to be addressed publicly.
Both the PALM007 and STOMP studies were NIH funded and it is not acceptable that full results have not yet been made publicly available.
- Siga Technologies press release. TEPOXX (tecovirimat) Approved in Japan for the Treatment of Orthopoxviruses. (2 January 2025).
www.globenewswire.com/news-release/2025/01/02/3003478/9738/en/TEPOXX-tecovirimat-Approved-in-Japan-for-the-Treatment-of-Orthopoxviruses.html - Tecovirimat fails to show benefit in randomised STOMP study in mpox clade 2. HTB (10 December 2024).
i-base.info/htb/49733 - Lack of tecovirimat activity against mpox clade 1. HTB (21 October 2024).
i-base.info/htb/49171

