bictegravir
1 January 2025. Related: ARVs and PrEP, Integrase inhibitors
no separate formulation

1 January 2025. Related: ARVs and PrEP, Integrase inhibitors
no separate formulation
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes

Biktarvy is a pink/grey film-coated tablet (15 mm x 8 mm) with “GSI” on one side and “9883” on the other.
1 October 2025. Related: ARVs and PrEP, Integrase inhibitors, NNRTIs
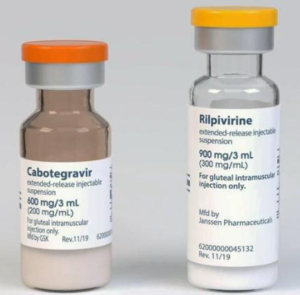
(not to scale)
Cabotegravir-LA and rilpivirine-LA are long acting injections given by intramuscular injections every two months.
Full details about cabotegravir LA + rilpivirine LA (Vocabria+Rekambys or Cabenuva)
27 October 2025. Related: ARVs and PrEP, Integrase inhibitors, PrEP
Cabotegravir-LA (CAB-LA) is a long acting injection given by intramuscular injections every two months.
30 August 2024. Related: ARVs and PrEP, Integrase inhibitors
![]()
Dolutegravir 50 mg is a 9 mm diameter round yellow tablet.
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes
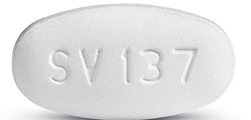
Dovato is a white tablet embossed with “SV137”. (Source: aidsinfo.nih)
1 May 2018. Related: ARVs and PrEP, Integrase inhibitors

Single drug formulations of elvitegravir are no longer used. Instead, elvitegravir is included with other drugs in fixed dose combinations.
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, NNRTIs, Nukes, PIs
A generic drug is one that is manufactured by a different pharmaceutical company to the company that invented the medicine.
12 April 2016. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes, PK booster
Full details about Genvoya: elvitegravir + cobicistat + emtricitabine + TAF (E/C/F/TAF)
12 May 2018. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, NNRTIs
![]()
3 January 2022. Related: ARVs and PrEP, Integrase inhibitors


Once-daily (600mg) Twice-daily (400 mg)
4 January 2022. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes
![]()
Full details about Stribild (elvitegravir + cobicistat + emtricitabine (FTC) + tenofovir DF)
1 January 2025. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, NNRTIs
Picture not included as there are many different versions.
Full details about TLD or LTD (tenofovir disoproxil, lamivudine, dolutegravir)
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes
