The tuberculosis vaccine pipeline
14 July 2011. Related: Pipeline report, TB coinfection.
Claire Wingfield and Richard Jefferys
The Bacille Calmette-Guèn (BCG) vaccine provides protection from the most severe forms of paediatric tuberculosis (TB) disease, saving the lives of an estimated 40,000 children each year. BCG is a valuable tool in combating child morbidity and mortality and is included in the World Health Organization (WHO) Expanded Programme on Immunization (EPI), but it is not sufficient to eliminate TB as a public health threat because it offers incomplete protection. Most important, BCG cannot prevent pulmonary TB – the most common form of the disease – and it is not recommended for use in HIV-positive infants because it can cause a potentially deadly immune reaction. A vaccine to provide lifetime protection against all forms of TB in all populations will be essential in eliminating TB. A novel vaccine that is only 60% effective could reduce TB incidence approximately 80% by 2050 (Abu-Raddad 2009).
TB vaccine development is resource- and time-intensive. Because vaccine studies must show that they are able to reduce TB incidence on a population level they take longer and require many more participants than do treatment and diagnostic trials. The search for a new TB vaccine has been excruciatingly slow despite the desperate global need, but recent developments offer encouraging signs of progress. Ten novel vaccine candidates are in clinical trials and there is a robust pipeline of constructs in preclinical studies thanks to the efforts of a relatively small but committed community of researchers, funders, and advocates.
Who is involved in developing new TB vaccines?
A handful of nongovernmental organizations, universities, and research institutions from the public and private sector are driving TB vaccine development. The South African TB Vaccine Initiative (SATVI), the European and Developing Countries Clinical Trials Partnership (EDCTP), the Tuberculosis Vaccine Initiative (TBVI), and Aeras are playing key roles in almost every TB vaccine trial. This reveals the limited infrastructure available for clinical TB vaccine research. SATVI is the only institution that currently has the expertise and capacity to conduct large-scale phase III efficacy studies and recently completed enrollment of the first efficacy trial in infants in more than 80 years. The EDCTP is playing a key role in facilitating TB vaccine research by establishing ?networks of excellence.?The TBVI and Aeras are advocating for increased resources and working with regulators to clarify the pathway for a new vaccine. Each of these organizations is working to build TB vaccine research infrastructure but all remain underresourced in comparison to the needs they are attempting to address. A combination of large pharmaceutical and smaller biotechnology companies, universities and government institutions are conducting the basic science and clinical research that keeps the pipeline filled and moves existing candidates forward.
The vaccine clinical pipeline
Ten vaccine candidates are currently being evaluated in phase I and II clinical trials. Although as of June 2011 twelve constructs are listed in the Working Group on New TB Vaccines? pipeline, one is inactive (M. smegmatis), and a phase III study of M. vaccae has been completed but studies results must be confirmed and yet no further studies of the vaccine are planned at this time.
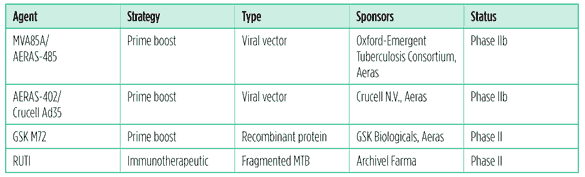
Table 1. TB vaccine constructs in phase II clinical trials (as of July 2011)
MVA85A/Aeras 485
MVA85A/AERAS-485?a recombinant attenuated version of the vaccinia virus (cowpox) combined with TB antigen 85A?is the most clinically advanced TB vaccine to date. The vaccine was developed at Oxford University and is being evaluated as a booster of preexisting immune responses to antigen 85A – which are present in most people either as a result of BCG vaccination or natural exposure to TB.
A total of twelve clinical trials of MVA85A/AERAS-485 have been completed and four are ongoing. Phase I and II safety studies indicate that the vaccine is well tolerated, with no serious adverse events. A trial in infants given the vaccines recommended in the WHO EPI showed no negative impact, but the EPI vaccines did slightly reduce the magnitude of the immune responses induced by MVA85A. Researchers think that this may be because the adjuvants – substances that stimulate an immune response to antigens in the vaccine – used in the EPI vaccines preferentially enhance the antibody Th2 immune response and thereby diminish the cellular Th1 response favored by MVA85A (McShane 2010). Aeras has partnered with the Oxford-Emergent Tuberculosis Consortium Ltd. (OETC) on a phase IIb efficacy trial of this candidate in infants that completed enrollment in April 2011. A second phase IIb efficacy trial in HIV-positive adults is due to begin later this year (Woolley 2011).
The OETC, a joint venture between the University of Oxford and Emergent BioSolutions Inc., has the rights to fully commercialise the vaccine, and Aeras will have the rights to distribute the vaccine to resource-limited populations for humanitarian purposes.
AERAS-402/Crucell Ad35
AERAS-402/Crucell Ad35 is one of two adenoviral-vectored vaccines in the TB vaccine pipeline. The vaccine is a replication-deficient adenovirus 35 (Ad35) that serves as a viral vector?a virus modified to deliver TB genetic material?for DNA-expressing TB antigens 85A, 85B, and 10.4. Adenoviruses are potent inducers of CD8 T-cell responses, which are considered important for developing an effective vaccine-induced immune response. This construct is being developed by Aeras and Crucell NV?a Dutch biopharmaceutical company with a particular focus on developing adenovirus-based vaccine vectors for infectious diseases.
When given after priming with BCG in adults, AERAS-402/Crucell Ad35 has been shown to induce polyfunctional CD4 T-cells and strong CD8 T-cell responses, suggesting it may have potential as an immunotherapy (Sadoff 2010). A phase II proof-of-concept clinical trial in HIV-negative infants ages 16?26 weeks is ongoing. The study includes an initial dose-finding period, followed by a safety and efficacy phase that will recruit over 4,000 infants (ClinicalTrials.gov 2011d). A phase II trial evaluating the safety and immunogenecity of AERAS-402/Crucell Ad35 in HIV-infected, BCG-vaccinated adults with greater than 350 CD4 cells was initiated in 2009. It is currently paused to further enrollment pending funding considerations (Leadman 2011).
GSK M72
GlaxoSmithKline (GSK) is working with Aeras to conduct phase II studies of GSK M72, a recombinant protein vaccine with an adjuvant. Early results show that the vaccine is well tolerated clinically and produces a measurable immune response. The vaccine has been studied with several of GSK’s proprietary adjuvants, with a compound named AS01E eventually selected for further development. GSK M72 is a vaccine that is made up of an adjuvant and two recombinant TB proteins meant to strengthen the immune response to two fragments of the TB bacterium that are commonly recognised by the immune system. The vaccine has induced robust polyfunctional CD4 cell responses against the M72 antigen, but no CD8 cell responses. No serious adverse events have occurred; the main side effects are transient local injection site reactions (Ofori-Anyinam 2010). A phase II study assessing the safety and immunogenicity in HIV-positive adults with or without ART in TB endemic areas is underway (ClinicalTrials.gov 2011c).
RUTI
RUTI is a killed TB vaccine that was originally discovered at Institut Germans Trias i Pujol and is now being developed by the biotech company Archivel Farma. The vaccine is being evaluated for its potential to accelerate the treatment of latent TB infection in combination with isoniazid (Ruiz 2010). The WHO recommends six months of daily isoniazid as a standard of care to treat latent TB infection and prevent progression to active TB disease. The preclinical data suggest that the RUTI vaccine plus isoniazid for one month may be as effective as six months of isoniazid (Churchyard 2010). A phase II study that compared three different doses of RUTI plus one month of isoniazid to six months of isoniazid plus placebo in HIV-positive and HIV-negative adults has been completed (ClinicalTrials.gov 2011a). Final results are pending. If this regimen proves to be as effective as the standard of care for latent TB infection it might be preferable for TB programmes because of the reduction in duration of therapy and potential for reduced risk of isoniazid resistance.
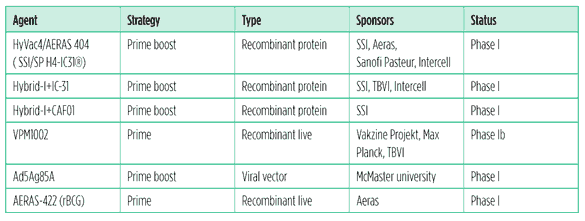
Table 2. TB vaccine constructs in phase I clinical trials (as of July 2011)
HyVac4/AERAS 404, Hybrid-I+IC-31, Hybrid-I+CAF01, and SSI H56-IC31
The Statens Serum Institute (SSI), a Danish research institution, has discovered key antigens and developed a number of technologies that are important for the development and production of a new TB vaccine. The SSI currently has three subunit protein vaccines combined with adjuvants in human testing: HyVac4/Aeras 404, Hybrid-I+IC-31, and Hybrid-I+CAF01. The SSI is partnering with Aeras, TBVI, Intercell (a biotech company), and Sanofi Pasteur (the vaccine division of the pharmaceutical company Sanofi-Aventis) to develop these constructs.
HyVac4/Aeras 404 also referred to as SSI/SP H4-IC31, uses SSI’s H4 antigen (a fusion protein of 85B and 10.4) combined with Intercell’s IC31® adjuvant to stimulate T-cell mediated immunity. Aeras and the SSI entered into a development partnership for H4-IC31 in 2005. In 2008, the SSI partnered with Sanofi Pasteur to further develop this candidate. It has undergone three phase I clinical trials in adults and Aeras is currently conducting a phase I trial to test this candidate in healthy adults (Leadman 2011).
Hybrid1, containing the TB antigens 85B and ESAT6, is combined with either IC31 or CAF01 adjuvants (Hoff 2010). All are being developed as booster vaccines and have completed safety studies in humans.
SSI has published promising preclinical data on an additional candidate that include a novel latency-associated TB antigen, Rv2660c, along with Ag85B, ESAT-6 and the IC31 adjuvant (Aagaard 2011). Dubbed SSI H56-IC31®, this vaccine is now poised to undergo phase I testing in humans in a collaboration with Aeras supported by the Bill and Melinda Gates Foundation Grand Challenge #12 (GC#12) consortium.
VPM1002
VPM1002 is a live vaccine made from a genetically modified BCG strain. The vaccine was originally created by the Max Planck Institute for Infection Biology and is now being developed by the company Vakzine Projekt Management. The vaccine has induced TB-specific immune responses, and is being developed as a priming vaccine (Grode 2010). A phase Ib trial is currently underway that evaluates safety, tolerability, and immunogenicity of three doses of VPM1002 in healthy adults using standard BCG immunization as a comparator (ClinicalTrials.gov 2011b).
Ad5Ag85A
Ad5Ag85A is the other adenoviral-vectored vaccine in the pipeline, and uses adenovirus 5 (Ad5).It is being evaluated as a BCG prime/boost vaccine.The developers at McMaster University are interested in pursuing intranasal delivery (Xing 2010). Phase I safety and immunogenicity study in BCG-vaccinated and nonvaccinated healthy adults is underway (ClinicalTrials.gov 2011e).
AERAS 422 (rBCG)
Aeras has developed a recombinant BCG priming vaccine currently undergoing evaluation in a phase I clinical trial in BCG-naive adults. AERAS-422 has been modified with an endosome escape mechanism and over-expresses three key TB proteins 85A, 85B and Rv3407 to elicit a greater protective immune response in the body. A second phase I trial of AERAS-422 will start later in 2011 (Leadman 2011).
Recommendations
There is overwhelming agreement that a safe, tolerable, easy-to-administer vaccine that provides lifetime protection against all forms of TB infection and disease, in all populations and age groups, will be key to reaching the goal of eliminating TB by 2050. However, few seem willing to pay for the research and development required. The Global Plan to Stop TB 2011-2016 estimates what will be needed to develop new tools to prevent, diagnose, and treat TB.
The direct costs to develop one TB vaccine candidate for one target population could be as much as US$315 million. The Global Plan estimates that US$1.9 billion will be needed between 2011 and 2015 in order to have three vaccine candidates in phase III efficacy trials (Stop TB Partnership 2010). The costs of developing a new vaccine include investment in preclinical and basic science research to better understand how the immune system responds to TB and to replenish the pipeline.
Resources need to be dedicated to manufacturing the vaccine and building the capacity of clinical trial sites to conduct later-stage trials that are larger and more complex. As a vaccine trial nears regulatory approval, advocacy is needed to clarify regulatory pathways and create informed community and provider demand. Annual TB vaccine funding must reach US$250 million in 2011 and nearly US$440 million in 2015 to develop and introduce a vaccine effective against all forms of TB and for all age groups including people with and without HIV (Stop TB Partnership 2010).
Yet TB vaccine research funding – representing 18% of overall TB R&D investments – only reached US$108.8 million in 2009. The Bill and Melinda Gates Foundation is the leading funder in this research area, though its contribution declined by 40% from US$66.9 million in 2008 to US$47.6 million in 2009, and the US National Institutes of Allergies and Infectious Diseases (NIAID), the second largest funder of TB vaccine research, flatlined its contribution (Salazar 2011). If this trend continues, it will derail progress and stall new developments. Current funders must increase their investments, and middle-income countries with high TB burdens – like Brazil, China, India, Russia, and South Africa – must commit resources to the search for a new TB vaccine.
Civil society needs to demand a better and safer TB vaccine. HIV-positive infants who are at increased risk for developing more severe forms of TB disease are unable to benefit from BCG’s limited protection. Communities must participate as more than study volunteers through creating demand and advocating for their governments to invest in research and TB programmes to rapidly scale up more effective vaccines. HIV treatment literacy campaigns have shown that an engaged and informed civil society is critical to accelerating research, mobilising resources, and strengthening the national response. Advocates need to educate themselves about the gaps in TB control, understand how research can help to address them, and demand action.
Conclusion
After languishing for many years, the search for an effective TB vaccine is finally gaining momentum. With ten vaccines in clinical trials, the pipeline is the fullest it has ever been. But this progress is threatened by lack of resources and infrastructure. If these needs are not addressed, the goal of eliminating TB as a public health threat by 2050 will not be reached.
References
Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 2011;17(2):189?94. Epub 2011 Jan 23.
Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM Jr., Dye C, Halloran ME 2009. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proceedings of the National Academy of Sciences USA 2009;106(33):13980?85.
Churchyard G 2010. Personal communication, 12 December 2010.
ClinicalTrials.gov 2011a. Clinical trial to investigate the safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI® following one month of isoniazid treatment in subjects with latent tuberculosis infection. Retrieved 27 May 2011 from
http://clinicaltrials.gov/ct2/show/NCT01136161.
ClinicalTrials.gov 2011b. Dose-escalation study on safety and immunogenicity of VPM1002 in comparison to BCG in healthy volunteers in South Africa. Retrieved 27 May 2011 from
http://clinicaltrials.gov/ct2/show/NCT01113281.
ClinicalTrials.gov 2011c. Safety and immunogenicity study of a candidate tuberculosis vaccine in human immunodeficiency virus (HIV )?positive adults. Retrieved 25 May 2011 from
http://clinicaltrials.gov/ct2/show/NCT01262976.
ClinicalTrials.gov 2011d. Study of Aeras 402 in healthy infants Retrieved 25 May 2011 from
http://clinicaltrials.gov/ct2/show/ NCT01198366.
ClinicalTrials.gov. 2011e. Study of the safety and immunogenicity of an adenovirus-based tuberculosis vaccine. Retrieved 25 May 2011 from
http://clinicaltrials.gov/ct2/show/NCT00800670.
Grode L 2010. SSI presentation at Second Global TB Vaccines Forum, Tallin, Estonia, 21?24 September 2010.
Jim?z Salazar E. 2010. Report on tuberculosis research funding trends, 2005?2009. New York: Treatment Action Group, 2010.
Leadman, A. Personal communication, 15 June 2011.
McShane H 2010. MVA85A presentation at Second Global TB Vaccines Forum, Tallin, Estonia, 21?24 September 2010.
Ofori-Anyinam O 2010. M72 presentation at Second Global TB Vaccines Forum, Tallin, Estonia, 21?24 September 2010.
Ruiz L 2010. RUTI presentation at Second Global TB Vaccines Forum, Tallin, Estonia, 21?24 September 2010.
Sadoff J 2010. AERA-402/Crucell Ad35 presentation at Second Global TB Vaccines Forum, Tallin, Estonia, 21?24 September 2010.
Stop TB Partnership 2010. The Global Plan to Stop TB 2011?2016. Geneva: Stop TB Partnership, 2010. Retrieved 6 June 2011 from
http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf.
Woolley J 2010. Personal communication, 26 May 2011. Xing Z 2010. Ad5Ag85A presentation at Second Global TB Vaccines Forum, Tallin, Estonia, 21?24 September 2010.