The tuberculosis treatment pipeline
14 July 2011. Related: Pipeline report, TB coinfection.
Claire Wingfield
It has been over 50 years since rifampin (also referred to as rifampicin) was synthesised. It was the first compound from a new class of anti-tuberculosis drugs called rifamycins, and remains one of the most powerful in its class. Two more anti-TB rifamycins were subsequently developed – rifabutin and rifapentine – but no new classes of anti-TB drugs have been approved since then. The discovery and introduction of rifampin marked the end of a busy two decades in TB drug development. After the breakthroughs made between the 1940s and 1960s TB drug development languished until outbreaks of TB resistant to the most powerful TB drugs – isoniazid and rifampin – coincided with the rise of the HIV epidemic in the early 1990s. These events cast a harsh light on the flaws in current TB treatment strategies – long duration of treatment, high pill burden, side effects, and treatment adherence issues – and led to a resurgence of interest in developing better, simpler, and shorter treatment regimens.
Over the past decade the TB treatment pipeline has returned from near death. Six new compounds from existing and novel drug classes are being evaluated in clinical trials with more in preclinical studies. Existing drugs that have been used off-label to treat TB and other bacterial infections are being repurposed to shorten treatment duration, reduce adverse events, and improve treatment outcomes. Two compounds from novel drug classes may be considered for regulatory approval in the coming year. Despite this progress we must remain only cautiously optimistic because while the pipeline is the most promising it has been in decades, it is still insufficient to eliminate TB as public health threat.
Concerns about small profit margins have deterred many large pharmaceutical companies from investing in TB drug development. As a result the TB treatment research community is small and consistently underfunded, with only a handful of companies engaged. Public sector research institutions and academia conduct much of the basic science research to fill the pipeline and work with product developers to conduct clinical studies. But TB researchers are resilient and resourceful and, as this year’s treatment chapter will show, they have done a lot with a little.
Latent TB infection
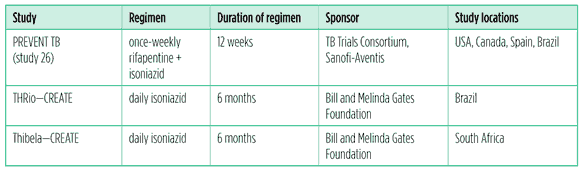
Every one of the two billion persons who are latently infected with TB is a potential future case of TB disease. Therefore, treatment of latent TB infection (LTBI) is critical to reducing the pool of new cases. The World Health Organization (WHO) recommends six to 12 months of daily isoniazid – one of the backbone drugs of TB treatment – particularly for people with HIV and children who are five years of age and younger (World Health Organization 2010b). Isoniazid preventive therapy (IPT) has long been a controversial issue in TB control. There is a mountain of clinical evidence proving the effectiveness of IPT in preventing the progression of latent infection (when a person’s immune system is able to control TB) to active disease (when a person is sick and able to transmit TB to others). But IPT is for healthy people, and therefore it is difficult to ensure treatment adherence. Ruling out active disease may be difficult if the patient is immunocompromised or has HIV and low CD4 counts. In many countries clear guidelines for programmatic implementation of IPT do not exist. Several large-scale studies are underway that attempt to gather evidence on how best to scale-up IPT in HIV-prevalent settings. Alternative preventive treatments which may be easier to adhere to or shorter in duration are being evaluated as potential replacements for IPT. See Table 1.
Table 1: LTBI studies as of July 2011
Rifapentine has a long half-life, allowing for intermittent dosing, and has been shown to have superior bactericidal activity to rifampin and rifabutin (Heifets 1990). TheTB Trials Consortium (TBTC), an international research network funded by the US Centers for Disease Control and Prevention (CDC), has been working with Sanofi-Aventis – the pharmaceutical company that makes rifapentine – to evaluate it in treatment-shortening regimens for LTBI and active disease.
The TBTC has been conducting TB treatment trials since the 1990s and recently completed the PREVENT TB trial – also referred to as TBTC study 26 – which evaluated whether giving rifapentine with isoniazid can shorten LTBI treatment. The study compared 12 weeks of once-weekly isoniazid and rifapentine to nine months of daily isoniazid. It showed that the rifapentine and isoniazid regimen was as effective as the standard self-administered nine-month daily regimen of isoniazid, and had better completion rates (Centers for Disease Control and Prevention 2011). Final safety data are pending for people with HIV and children between the ages of 2 and 12 years, and should be available by the end of 2011.
Because this study was conducted in low- and middle-TB-burden settings, further studies evaluating its effectiveness and tolerability in high-burden countries are needed – particularly those with high HIV prevalence. Each rifapentine and isoniazid dose was directly observed, so the recommendations for this regimen will include directly observed therapy (DOT) as the preferred method of administration. Recognising that this is not ideal for all programmes and patients, the TBTC is planning a study to compare adherence rates using DOT or self-administration with and without electronic reminders.
TB programmes in low-burden countries like the United States – where much of TB control is geared toward treating LTBI – are already considering this 12-week regimen. The CDC is expected to issue interim recommendations for the use of this new regimen in the United States later this year. The potential for a once-weekly regimen with shortened duration may not only improve adherence but also be cost effective by reducing patient visits, staff time, and number of pills being taken. Whether this can be reproduced in high-burden countries is unknown until studies are conducted. Until there is more data IPT remains the best option for treating LTBI in high-TB-burden settings. Two major studies evaluating population level scale-up of IPT in high-HIV and -TB settings are nearing completion and have already strengthened roll-out of IPT in Brazil and South Africa.
The Consortium to Respond Effectively to the AIDS/TB Epidemic (CREATE) – a group of research institutions based in Brazil, South Africa, the United States and Zambia – is wrapping up two studies evaluating the impact of programmatic implementation of mass IPT on TB incidence. Final analysis from the THRio study, which evaluated the provision of IPT among HIV positive persons attending HIV clinics in Brazil, is completed and will be released in July 2011. The final data from the Thibela study, which evaluated mass, mineshaft-wide IPT in South African gold mines, will be released in 2012. Already both studies have led to changes in national guidelines and clinical practice.
In Brazil, CREATE study data were cited as the rationale for expanding integration of IPT with ART and placing responsibility for IPT on the national AIDS programme (NAP). In late 2009, the NAP promulgated a policy requiring HIV clinics to take responsibility for screening patients for active TB and providing IPT to patients testing positive with a tuberculin skin test (TST).The NAP has included IPT and TB drugs in the SICLON, the system that controls drugs used to treat HIV and HIV-related opportunistic infections. This is an important step because it means that TB prevention and treatment will have the same status, availability, and control in HIV clinics nationwide as all the other medications (Eldred 2011). Likewise, in South Africa, the Thibela team gave substantial input into national department of health guidelines, making South Africa the first country to adopt the WHO’s recommended four-symptom TB screening tool (Eldred 2011).
Isoniazid preventive therapy is widely recognised as the standard of care for treating LTBI, but it is not an option for people who are latently infected with drug-resistantTB (DR-TB).Treatment of close contacts of DR-TB cases is usually based on anecdotal evidence and drug availability. The AIDS Clinical Trials Group (ACTG) – a research network that is funded by the US National Institutes for Health (NIH) – and the TBTC are considering a study that would evaluate the efficacy and tolerability of TMC207 (bedaquiline) compared with INH. ACTG study 5300 will compare TMC207 to INH for preventing TB disease in those 13 years and older who have household contact with persons with confirmed DR-TB. A number of factors must be addressed before this study can start, including approval from the company to begin to study the compound for this purpose before it is approved for treatment of active TB. It is promising that DR-TB is finally being considered as critical to LTBI clinical research.
Active disease
Current standard treatment for drug-susceptible TB (DS-TB) – TB bacteria susceptible to all first-line drugs – has shown a 95% cure rate. Unfortunately, many TB programmes are understaffed and poorly funded, therefore the majority of TB patients access care in settings that are vastly different from the tightly controlled environment of a research study. Actual cure rates can be as low as 57% in some high-burden countries (World Health Organization 2010a). The standard of care for DS-TB is two months of a four-drug combination – isoniazid, rifampin, ethambutol, and pyrazinamide – followed by four months of a isonizid and rifampin (or 2HRZE/4HR). This six-month regimen requires daily dosing and is often given as DOT, which is labor-intensive for both patient and provider. A number of studies underway are using existing compounds to reduce the length of first-line treatment to improve adherence rates.
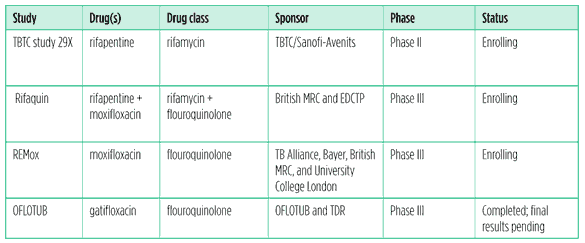
Table 2: Existing TB drugs in clinical studies for DS-TB as of July 2011
Repurposing existing compounds
Drug-susceptible TB
The TBTC has been evaluating the antimicrobial activity and safety of daily rifapentine for potential future use in treatment-shortening regimens. The TBTC’s study 29 evaluated the use of rifapentine 600mg given five days a week in place of rifampin in the context of standard therapy with isoniazid, pyrazinamide, and ethambutol during the eight-week intensive phase of first-line TB treatment. The study found no significant difference in the efficacy between the two regimens, whether defined as culture conversion rates at eight weeks (the primary endpoint) or time to culture conversion. Rifapentine administered according to the study protocol was safe and well tolerated. A notable finding in the study was that African site volunteers had lower concentrations of rifapentine compared to non?African site volunteers despite receiving the same dosages of the study drug. This is not fully understood, but may be partly due to the enhancing effect of food consumption on rifapentine concentrations. African patients in study 29 had fasted before taking rifapentine. The low rifapentine concentrations may also be due to pharmocogenomic differences – influence of genetic variation on drug response – between African and non-African participants, but further studies are needed to confirm this. The TBTC will be conducting a double-blind dose-ranging study in patients with drug-susceptible pulmonary tuberculosis to determine the safety and tolerability of rifapentine taken at 10 mg/kg, 15 mg/kg, or 20 mg/kg with food for seven days a week during the intensive phase of therapy.
Several studies underway are using flouroquinolones to shorten the duration of treatment from six to four months. Flouroquinolones are a class of broad-based antibiotics used to treat many bacterial infections. They have been used as part of second-line treatment for multidrug-resistant TB (MDR-TB) but are not licensed for TB. The drugs in this class – levofloxacin, ofloxacin, moxifloxacin, and gatfloxacin – are completely cross resistant to one another.
The Rifaquin study – being conducted by the British Medical Research Council (MRC) with funding from the European and Developing Countries Clinical Trials Partnership (EDCTP) – is assessing whether rifapentine and moxifloxacin, when given together, can shorten first-line treatment and allow for intermittent dosing. It is a three arm study comparing the six-month standard-of-care regimen (2HRZE/4HR), versus two months of daily ethambutol, rifampin, and pyrazinamide plus moxifloxacin followed by two months of twice-weekly moxifloxacin and rifapentine (2EMRZ/2P2M2), versus two months of daily ethambutol, moxifloxacin, rifampicin, and pyrazinamide followed by four months of once-weekly moxifloxacin and rifapentine (2EMRZ/4P1M1). Recruitment is ongoing.
The Global Alliance for TB Drug Development (TB Alliance) is conducting the REMox TB trial in collaboration with University College London and the British MRC as part of its moxifloxacin for TB development programme with Bayer Healthcare. The trial is receiving major funding from the Bill and Melinda Gates Foundation, the EDCTP, and USAID and through the NIH which will be enrolling volunteers through the ACTG. This phase III trial is evaluating the use of moxifloxacin in place of ethambutol or isoniazid to shorten first-line treatment to four months. The study is currently enrolling in Africa, Asia, and Latin America, and is in the process of additional sites. Enrollment will be complete by the end of 2011, with final study results by 2014 (Ginsberg 2011).
Patient follow-up has been completed in the OFLOTUB consortium’s trial evaluating gatifloxacin as a replacement for ethambutol in a shortened first-line treatment regimen. Problems in data management have resulted in unexpected delays in data analysis. Safety and efficacy results are expected by the end of 2011 (Lienhardt 2011).
While it is encouraging to see so much research underway to improve treatment for DS-TB, children are absent from all of these studies. While effictiveness in children may be extrapolated from adults, safety and proper dosing cannot. But children are more susceptible than adults to rapid progression from exposure to infection and to severe disease. Therefore safety, tolerability and pharmacokinetic (PK) data – how a drug is absorbed, distributed, metabolised, and eliminated by the body – must be collected to establish safety profile and accurate dosing in children of all ages and development stages.
Paediatrics
Children have been excluded from TB treatment research for the most part and are not a priority for national TB programmes. Because of the difficulty in confirming a TB diagnosis in children using bacteriological methods such as sputum smear microscopy or culture, researchers and product developers are hesitant to conduct studies in children. Likewise, the focus on smear-positive TB, which is more contagious, means public health programmes often neglect young and HIV-infected children. There are considerable differences in national recommendations in paediatric drug dosing (Ramachandran 2011), and many children have been receiving sub-therapeutic levels of TB drugs. In 2010 the WHO issued Rapid Advice: Treatment of Tuberculosis in Children to provide a framework for accurate dosing of first-line treatments for children (World Health Organization 2010c). Literature reviews of PK and toxicity data in children have shown that, while the principles of treatment in children and adults are the same, the dosages are not (Graham 2010). Children metabolise drugs differently, and therefore the amount of drug given to them cannot just be scaled down from adult data (Ramachandran 2011).
The 2010 WHO guidelines recommend new dosages of isoniazid, rifampin, pyrazinamide, and ethambutol to account for these differences. Unfortunately, implementing these new recommendations is quite challenging for national programmes because the child-friendly formulations (e.g. crushable, dispersable, or scored tablets or capsules) of current single-dose drugs and fixed-dose combinations (FDCs) that are meant to ease dosing of multidrug regimens do not exist. Inclusion of children earlier in treatment research with prioritization of collecting PK and safety data is essential to the development of child-friendly treatment regimens for first- and second-line drugs. As a matter of urgency simple weight-band tables that can guide the dosages and schedules for single and combinations of current drugs like those used in paediatric ARV treatment are needed.
Table 3: Existing TB drugs in clinical studies for DS-TB as of July 2011
MDR-TB treatment
The International Union against Tuberculosis and Lung Disease (IUATLD) is sponsoring the Evaluation of a Standardised Treatment Regimen of Anti-Tuberculosis Drugs for Patients with MDR-TB (STREAM) tria. It will assess a nine-month standardised treatment regimen for MDR-TB that achieved excellent outcomes with a cure rate of 87% in a non-randomised observational study in Bangladesh (Van Deun 2010). Modeled on the Bangladesh regimen, the STREAM regimen uses moxifloxacin, clofazimine, ethambutol, and pyrazinamide for nine months, supplemented by prothionamide, kanamycin, and isoniazid during an intensive phase of four months. The aim of this study is to show that this shorter treatment regimen is at least as effective as the current lengthier treatments used throughout the world to treat MDR-TB. The British MRC is conducting this trial and is expected to begin enrollment in several sites in late 2011 and early 2012 (Ornstein 2011).
Last year’s report included a description of the TBTC’s LiMiT study (also known as study 30), a double-blind, placebo-controlled trial evaluating the safety and tolerability of low-dose, limited-duration linezolid – an oxazolidinone used off-label in treatment of DR-TB. The study closed enrollment in April 2010 and completed follow-up in September. Unfortunately, conclusions regarding the safety and tolerability of lower-dose linezolid will be limited because the investigators found evidence of sporadic, nonrandom irregularities in the distribution of the study drug to patients not in keeping with their treatment assignment. Failure to implement the protocol correctly jeopardises the validity of study data. The research team and TBTC are committed to additional analysis furthering the understanding of what happened and in sharing such knowledge with the broader research community.
Maternal TB
TB control is crucial to maternal and child health in TB-endemic areas. TB is the leading infectious cause of death in women (Gupta 2011).Women bear the greatest burden of HIV during their childbearing years, and the same applies to TB. Maternal TB/HIV coinfection is associated with high incidence of postpartum maternal and infant death (Gupta 2007) and increased risk of maternal transmission of HIV and TB (Gupta 2011; Mofenson & Laughton 2007). Yet there is a dearth of data guiding how to treat pregnant women with TB drugs. A recent observational study conducted in Iran followed six pregnant women diagnosed with MDR-TB and found that treatment with a standardised second-line regimen was safe and effective in curing maternal TB and preventing childhood TB (Tabarsi 2010). These results are encouraging, but only six volunteers participated in this study, so the findings are not generalizable. Many second-line drugs have not been evaluated during pregnancy and the evidence is weak for those – such as linezolid and streptomycin – contraindicated for use in this population. While conducting clinical trials in pregnant women may be challenging, it is imperative that research institutions and product developers conduct PK and safety studies in them to ensure that these drugs are used safely and effectively to prevent and cure maternal and childhood TB.
Novel and second-generation compounds
The Global Plan to Stop TB 2011-2015 estimates that US$700 million annually is needed to adequately fund TB treatment research over the next five years (Stop TB Partnership 2010). To reach this target, 2011 funding levels must more than triple. With the current global fiscal crisis, and with budget cuts looming for public-sector funders, it seems unlikely that this will happen. But as new compounds move through the pipeline private-sector investment is increasing. Just by continuing a phase II study of its new compound, Otsuka Pharmaceuticals became the leading funder of TB treatment research in 2009 ( Jim?z Salazar 2011). For the first time in decades there are promising drugs with novel mechanisms of action that may be considered for regulatory approval in the next year. These new drugs may revolutionise TB treatment in the not-so-distant future. See Table 4.
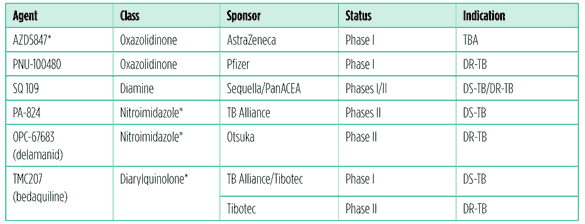
Table 4: Novel and second-generation compounds as of July 2011
Notes: *Indicates new drug class.
AZD5847
AstraZeneca Pharmaceuticals has completed two phase I safety, tolerability, and PK dose-escalation studies in healthy volunteers for its second-generation oxazolididnone, AZD5847. Proof of principle was demonstrated, since plasma concentrations exceeded the therapeutic exposures predicted by preclinical models at doses that are generally well tolerated. The detailed results will be presented in fall 2011. The compound will be moving into a phase IIa 14-day extended and early bactericidal activity (EBA) study in volunteers with DS-TB (Lawrence 2011).
PNU-100480
A multidose study of Pfizer’s second-generation oxazolidinone PNU-100480 in healthy volunteers found all doses of PNU-100480 (up to 600mg twice per day) to be safe and well-tolerated and that they exhibited superior bactericidal activity to linezolid – an earlier-generation oxazolidinone used as last resort drug for DR-TB. The first study in TB patients is anticipated to begin enrollment in June 2011. Pfizer intends to develop the compound for DR-TB (Wallis 2011).
SQ109
SQ109, a second-generation ethane diamine antibiotic, is the lead compound from Sequella. With collaborators from the Pan African Consortium for Evaluating Antituberculosis Agents (PanACEA), Sequella is evaluating SQ109 in a phase IIa early bactericidal study to determine optimal dosing in DS-TB. Phase II/III studies are expected to begin enrollment in 2012. In parallel, Sequella and the Maxwell Biotech Venture
Fund announced an agreement to develop SQ109 for DR-TB in Russia, Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Moldova, Tajikstan, Uzbekistan, and possibly Turkmenistan and Ukraine. (Horwith 2011).
Regulatory challenges
Regulatory rules and requirements vary from country to country or region. Approval from stringent regulatory authorities like the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) has traditionally been sufficient for countries with limited regulatory capacity to grant approval for new treatments, particularly for life-threatening conditions. However, there is limited regulatory experience in the TB field because no new drug class has been approved since the 1960s and regulatory science is much more demanding now than it was then. Requirements for regulatory approval for TB treatments are not harmonised across agencies; for instance, the EMA requires that drug developers submit a paediatric investigational plan and timeline for evaluating a new TB compound in children while the FDA does not. The lack of regulatory harmonization means sequential and/or parallel regulatory filings in high- and low-burden countries along with long review timelines and individual application requirements (Haaxaire-Theeuwes 2011). Even applying to conduct a study in a country may take up to one year to get a clinical trial approved. These administrative delays hinder implementation and raise the cost of studies, and may deter companies from investing in developing treatments for TB.
PA-824
PA-824 comes from a new class of drugs knows as nitroimidazoles, and is licensed by the TB Alliance from the former biotech company Chiron. PA-824 has been tested in two extended, dose-ranging EBA trials assessing the ability of doses from 50mg to 1200mg given daily for 14 days to kill TB in the lungs of newly diagnosed patients. Based on the results of these studies, a 200mg dose of PA-824 was selected for late-stage development as one component of a novel regimen to be tested for treatment of both DS- and DR- TB. Results from the first study were published in 2010. The results of the second EBA study will be published later in 2011 (Ginsberg 2011).
OPC67683 (delamanid)
Delamanid, formerly known as OPC67683, comes from the same class of drugs as PA-824; the two drugs are completely cross resistant to one another. Otsuka Pharmaceuticals is in the process of completing its analyses of data from a phase IIb study of delamanid plus optimised background therapy in volunteers with confirmed MDR-TB as well as drug-drug interaction (DDI) studies with ARVs. Plans for future clinical trials will follow the completion of the analysis of these trials. Delamanid neither induces nor suppresses the cytochrome P450 (CYP P450) enzymatic pathways; therefore, additional DDI studies are not planned in the near future. There are no plans for early or expanded access to delamanid until after the analyses of existing studies are complete (Carlevaro 2011).
Recently the company established Otsuka S.A., in Geneva, Switzerland, a new entity and subsidiary of Otuska Pharmaceuticals, which will serve as the company’s central operations for developing and implementing public health policies regarding access and capacity building, and corporate social responsibility programmes, in connection with its global TB programme. This commendable development suggests Otsuka is sensitive to the global issues posed by the likely advent of a new TB drug.
TMC207 (bedaquiline)
Tibotec (a subsidiary of Johnson & Johnson) has developed TMC207 – recently given the generic name of bedaquiline – the first compound from a new class of drugs called diarylquinolones. Final 24-week data from stage 2 of a phase II trial showed volunteers who added TMC207 to a standard background MDR-TB regimen had faster time to culture conversion and a higher number of culture conversions than in volunteers on standard MDR-TB treatment (McNeeley 2010). Stage 2 patients are being followed while they complete their background regimens.
The company is conducting DDI studies with ARVs known to inhibit CYP450. Coadministration with the boosted protease inhibitor lopinavir/ritonavir (LPV/r) increased exposure to TMC207 by approximately 20%. (van Heeswijk 2010). Results of the nevirapine interaction trial will be released in July 2011. ADDI study of TMC207 and efavirenz has been completed; final analysis is expected in mid-2011 (Dooley 2011).
Tibotec has finished recruitment at sites in Europe, Asia, and Africa for an open-label trial of TMC207. Adults with smear-positive, confirmed MDR-TB or extensively drug resistant TB (XDR-TB) are eligible, including people with HIV. Data will be available later in 2011. The company is currently in discussions with health authorities on the design of a phase III trial, planned to start in 2012. The paediatric investigational plan that will guide future clinical studies of TMC207 in children to establish safe and effective dosing based on age and development has been approved by the EMA and has been shared with the FDA (Haaxaire-Theeuwes 2011).
The TBTC and the NIH-funded IMPAACT network are hoping to collaborate with Tibotec to conduct a PK study of TMC207 in children of all ages. The trial would start with adolescents and work down to infants from birth to six months of age. Once data from HIV-positive adults become available, children with HIV would be included (Hessling 2011). This is contingent upon approval from Tibotec to use TMC207 preapproval.
Preapproval access to compounds
Expanded access and compassionate use programmes provide preregulatory-approval access to lifesaving treatments – like ARVs – that have demonstrated efficacy to patients who cannot participate in a controlled clinical trial. These programmes have been used to accelerate access to promising treatments for HIV and cancer but have never been implemented in the context of TB treatment. As promising new treatments for DR-TB advance through the pipeline it is important to provide access to them for people with limited to no treatment options – particularly people with XDR- or pre-XDR- TB. Between the submission of an application for regulatory approval and receiving it, the experimental drug is not accessible – regardless of the effectiveness of the drug – unless it is made available through an expanded access or compassionate use programme. One of the concerns about providing preapproval access to drugs is how to ensure that they are used properly so that resistance doesn?t develop to the drugs before they are made available to the general public. Some countries do not have a legal framework for compassionate use and therefore do not allow access to unlicensed drugs. Tibotec is the first company to provide access to its compound preapproval, and has initiated a compassionate use programme to provide access to TMC207 to XDR- or pre-XDR-TB patients who are ineligible to participate in any other TMC207 study. This programme is currently reviewing the first requests from health care providers. An expanded access trial is expected to begin in summer 2011.
Tibotec and TB Alliance are codeveloping TMC207 with Tibotec taking the lead for DR-TB, the TB Alliance taking the lead for DS-TB and the two organizations collaborating to discover ‘next generation’ diarylquinolines – the same drug class as TMC207. The TB Alliance conducted a DDI study with TMC207 and rifapentine and rifampin this year. Results are expected to be published in 2011. The TB Alliance in collaboration with the ACTG are planning a DDI study with rifabutin to determine the optimal approach for moving forward with a rifamyacin-based TMC207 regimen for DS-TB. Preliminary results from a 14-day EBA study indicate that all TMC207 dosing regimens evaluated were well tolerated and produced measurable bactericidal activity. Final results of this study will be available in late 2011. Both PA-824 and TMC207 are being developed by the TB Alliance as part of combination regimens rather than single drugs (Ginsberg 2011).
Regimen change
Although a combination of drugs is required to cure TB, drug development has traditionally evaluated one new compound at a time by adding an experimental drug to a standardised regimen. The FDA has expressed concern that this model of drug development is unethical given the risk for the emergence of resistance and rendering the new compound ineffective (Woodcock 2011), and has drafted Guidance for Industry Codevelopment of Two or More Unmarketed Investigational Drugs for Use in Combination (Food and Drug Adminitsration 2010) to facilitate the development of novel combination therapies rather than sequential drug development. The Critical Path to TB Regimen (CPTR) was established in 2009 to provide a forum for different stakeholders in TB research to work together to speed up the development of novel TB regimens. There are several challenges to this approach, not limited to different timelines of drug development, the hesitation of sponsors to work together and share data, and the lack of appropriate drug-drug interaction data to guide dosing regimens in such studies. But these challenges are not insurmountable and if successful these types of studies could reduce the time to regimen change from over 20 years to less than 10 years.
Table 5: Regimen change as of July 2011
NC-001
The TB Alliance has initiated the first novel TB treatment combination trial, NC-001. The primary objective of this trial is to evaluate the extended EBA, safety, tolerability, and PK of TMC207 alone, TMC207 plus pyrazinamide, PA-824 plus pyrazinamide, and PA-824 plus pyrazinamide and moxifloxacin, dosed daily over 14 days. PA-824 plus pyrazinamide and TMC207 plus pyrazinamide are promising building blocks for novel treatment-shortening regimens as they have been shown to be synergistic in a mouse model of TB. TMC207 plus PA-824, a combination that shows some antagonism in the same mouse model, is also being studied in NC-001 to evaluate whether it has potential in humans as a building block for a novel TB regimen for both DS- and DR-TB (Ginsberg 2011).
NC-001, initiated in early 2011, represents the first study in TB patients of a combination containing more than one new drug for TB; the novel three-drug regimen is PA-824 plus moxifloxacin and pyrazinamide. Enrollment into NC-001 has recently been completed. Results from this study are expected to be available by the end of 2011. Depending on the results of NC-001, a two-month treatment study of this three-drug regimen is being planned for initiation in early 2012 (Ginsberg 2011).
Recommendations
Current treatment strategies cannot eliminate TB as a public health threat by 2050. Treatment for active TB disease takes from six months to two years, requires patients to take multiple pills (in some cases at different times of day), and causes mild to severe (and potentially irreversible) side effects. Better drugs are needed, as are more data on how best to use current treatments in people with HIV and children who are at greater risk for disease progression and more severe disease.
Childhood TB
More than half of the ARVs approved to treat HIV have established simple weight band tables with paediatric dosing ranges and child-friendly formulations (Food and Drug Administration 2011). Meanwhile, there is a dearth of evidence guiding TB treatment for children (Burman 2008). Infants and young children bear a higher risk for TB disease progression. Pharmocokinetic and tolerability studies in children of all ages are desperately needed for current second-line drugs and new compounds in development. Once adult efficacy data has been established, paediatric PK and safety studies should be initiated to establish the optimal dose in children of all ages, starting with adolescents and then scaled down to infants. Likewise, manufacturers need to prioritise the development of FDCs and child-friendly formulations of current first-line and second-line drugs and new compounds for children. Without these formulations, the revised paediatric dosages will not be implemented and children will be denied the potential benefits of promising new drugs and regimens.
Maternal TB
TB remains a leading cause of death of women of childbearing age, yet few research institutions, product developers, and funders have prioritised this population. Maternal TB has a significant impact on the TB status and overall health of an infant. A pregnant woman who is coinfected with TB/HIV is 2.5 times more likely to transmit HIV to her newborn (Gupta 2011). If her TB remains untreated she is at risk for transmitting TB to her child in utero, during birth, or postpartum. It is imperative that mothers and their children be prioritised and included in TB treatment research.
Antiretroviral therapy as TB prevention
There are limited data on interactions between TB drugs and ARVs. Evidence continues to show the significant impact that ART has on reducing incidence and severity of TB among people with HIV, but there is very little information on how best to use current TB treatments and newer compounds with ARVs. Provision of ART is a critical intervention in preventing TB among people with HIV. Unfortunately, many people start ART at low CD4 counts, lessening the potential benefits from ART as TB prevention (Lawn 2011). Antiretroviral therapy must be scaled up in high-TB burden settings, and DDI studies with ARVs and new TB compounds are required to ensure that ART is included as an essential component of TB care.
Regulatory requirements
Regulatory authorities need to provide clear guidance to drug developers on requirements for conducting clinical trials, providing preapproval access to promising compounds, and applying for licensure. These requirements should be harmonised or at least synergised as much as possible with other national regulatory agencies to avoid unnecessary delays, added costs, and missed opportunities for accessing lifesaving treatments.
TB control
Cure rates for drug-susceptible TB may reach 95% in the best-functioning health systems, but the majority of TB patients are accessing their care in resource-limited settings where drug stockouts are not uncommon, not all drugs are quality assured, and staff are overwhelmed by the patient load and unable to provide adequate adherence support. As a result, many patients may find themselves relapsing or failing treatment because they did not complete their regimen or were not given the appropriate treatment. Countries need to commit to providing a consistent supply of quality-assured first- and second- line drugs and invest in developing and sustaining human resources required to run a functioning TB programme.
Conclusion
Over the past decade,TB treatment research has seen greater investment from the public and private sector and has produced a number of promising advances. But more investment is needed to ensure that we are able to build on this foundation and revolutionise treatment of TB. Major funders like the NIH, the Bill and Melinda Gates Foundation, and pharmaceutical companies must not scale back on their contributions, and high-burden countries like Brazil, India, Russia, China and South Africa must increase their investments. The potential to shorten treatment and dramatically improve outcomes for latent TB infection and active disease is close and demands commitments be kept and innovation encouraged if TB is going to be curable for all people.
References
Burman WJ, Cotton MF, Gibb DM, et al. 2008. Ensuring the involvement of children in the evaluation of new tuberculosis treatment regimens. PLoS Med. 2008;5(8):e176. doi: 10.1371/journal.pmed.0050176.
Carlevaro P. 2011. Personal communication, 13 June 2011.
Centers for Disease Control and Prevention. Press release: PREVENT TB: Results of a 12-dose, once-weekly treatment of latent tuberculosis infection (LTBI). 16 May 2011.
Dooley K. 2011. Personal communication, 18 May 2011.
Dorman, S. 2011. S29 update and results. Presentation at the TB Trials Consortium 29th Semi-Annual Group Meeting, Denver, CO, 13?14 May 2011.
Eldred L. 2011. Personal communication, 11 May 2011.
Food and Drug Administration. 2011. Approved antiretroviral drugs for pediatric treatment of HIV infection;
http://www.fda.gov/ ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118951.htm, accessed 24 April 2011.
Food and Drug Administration DRAFT guidance for industry codevelopment of two or more unmarketed investigational drugs for use in combination. Bethesda, MD:US Food and Drug Administration, 2010
Ginsberg A. 2011. Personal communication, 23 May 2011. Graham S. 2010. Treatment of paediatric TB: Revised WHO guidelines. Paedat Respir Rev. 2010. doi: 10.1016/j.prrv.2010.09.005.
Gupta A, Bhosale R, Kiniker A, et al. 2011. Maternal Tuberculosis: A Risk Factor for Mother-to-Child Transmission of Human Immundeficiency Virus. Journ Infect Dis. 2011;203:358?63.
Gupta A, Nayak U, Ram M, et al. 2007. Postpartum Tuberculosis Incidence and Mortality among HIV-Infected Women and their Infants in Pune, India, 2002?2005. Clinical Infectious Diseases 2007. 45:000?000.
Haaxaire-Theeuwes, M. 2011. Personal communication, 15 May 2011.
Heifets LB, Lindholm-Levy PJ, and Flory MA . 1990. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990. 141:626?30.
Hessling A. 2011. Personal communication. 21 May 2011.
Horwith G. 2011. Personal communication, 10 May 2011.
Jim?z Salazar E. 2011. 2010 report on tuberculosis research funding trends, 2005?2009. 2nd ed. New York: Treatment Action Group.
Jindani A. 2011. Personal communication, 10 June 2011.
Lawn SD, Harries AD, Williams BG, et al. 2011. Antiviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis. 2011;15(5):571?81.
Lawrence C. 2011. Personal communication, 2 June 2011. Lienhardt C. 2011. Personal communication, 16 May 2011.
McNeeley D, Diacon AH, Pym A, et al. 2010. TMC-207 versus placebo plus OBT for the tretreatment of MDR-TB: A prospective clinical trial. Abstract presented at the 41st World Lung Conference, Berlin, 11?14 November 2010.
Mofenson L & Laughton B. 2007. Human Immunodeficiency Virus, Mycobacterium Tuberculosis, and pregnancy: A deadly combination. Clin Infect Dis. 2007;45(2):250?53. doi: 10.1086/518975.
Ornstein T. 2011. Personal communication, 20 May 2011.
Ramachandran F, Hemanth Kumar AK, Swaminathan S. 2011. Pharmacokinetics of anti-tuberculosis drugs in children. Indian J Pediatr. 2011;78(4):435?42.
Schechter M, Zajdenverg R, Falco G, et al. 2006. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med. 2006;173:922?26.
Stop TB Partnership. The Global Plan to Stop TB 2011?2016. Geneva: Stop TB Partnership 2010;
http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf.
Tabarsi P, Moradi A, Baghaei P, et al. 2011. Standardised second-line treatment of multidrug-resistant tuberculosis during pregnancy. Int J Tuberc Lung Dis. 2011;(4):547?50.
Food and Drug Administration. 2011. Approved antiretroviral drugs for pediatric treatment of HIV infection;
http://www.fda.gov/ ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118951.htm, accessed 24 April 2011.
Food and Drug Administration DRAFT guidance for industry codevelopment of two or more unmarketed investigational drugs for use in combination. Bethesda, MD:US Food and Drug Administration, 2010.
Van Deun A, Maug AK, Salim MA, et al. 2010. Short, highly effective and inexpensive standardised treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684?92.
VanHeeswijkR,VandevoordeA,MeyvishP,etal.2010.Theeffectoflopinavir/ritonavironthepharmacokineticsofTMC207,aninvestigational mycobacterial agent. Abstract WEPE0097 presented at the 18th International AIDS Conference, Vienna, 18?23 July 2010.
Wallis RS. 2011. Personal communication, 18 May 2011.
Woodcock J, Griffin JP, Behrman RE. 2011. Development of Novel Combination Therapies. NEJM 2011; 364(11):985?87.
World Health Organization 2010a. Global tuberculosis control report 2010. Geneva, Switzerland: World Health Organization, 2010.
World Health Organization 2010b. Guidelines for the intensified case-finding and isoniazid preventive therapy for people living with HIV/AIDS in resource-constrained settings. Geneva, Switzerland: World Health Organization, 2010.
World Health Organization 2010c. Rapid advice: Treatment of tuberculosis in children. Geneva, Switzerland: World Health organization, 2010.