Structured treatment interruptions and treatment intensification
16 September 2000. Related: Treatment strategies, CROI 7th (Retrovirus) 2000.
Stefano Vella, MD, AIDS Clinical Care, July 2000.
With the realization that eradication of HIV from an infected individual is not an attainable objective at present, antiretroviral treatment has been redirected toward a less ambitious but very strategic goal: the long-term management of a chronic disease.
The best time to initiate antiretroviral therapy and the best initial regimen remain controversial. In order to prevent progressive immune damage, treatment ideally should be initiated as early as possible following infection. The attractiveness of early treatment, however, must be balanced against the drawbacks of prolonging the overall duration of therapy. Indeed, we know that the risk of disease progression is low until substantial CD4-cell count loss has occurred and that immune recovery is impressive even in patients with advanced infection.
Once the decision to start antiretrovirals has been made, however, the ultimate treatment goal should be maximal suppression of HIV replication, because any continuing replication carries the serious short-term risk of spurring the development of drug resistance. Several strategies are now available to achieve viral suppression, especially for persons initiating therapy. Regimens are usually composed of 3 or 4 drugs: 2 NRTIs combined with 1 or 2 PIs or an NNRTI. Regimens consisting of 3 NRTIs are currently being tested.
The superiority of any one of these potent initial regimens over the others has not yet been demonstrated, and sweeping recommendations are not possible. Because future options may be severely compromised by an initial regimen that is insufficiently potent or difficult to comply with, however, individual treatment decisions should be based on the strength of supportive data, tolerability of the regimen, potential for adverse effects, likelihood of important pharmacokinetic interactions, convenience and likelihood of adherence, and potential for subsequent treatment options should the regimen fail. Indeed, treatment failure after a variable amount of time is, unfortunately, quite common.
Reasons are multifactorial and include insufficient potency of the regimen, poor pharmacokinetics, poor adherence, and pre-existing resistance mutations. Given the expected benefits of suppressing viral replication for as long as possible, new therapeutic strategies are needed to maximize the long-term effectiveness of antiretroviral treatments. However, recent studies have suggested that induction maintenance therapy is not as effective as researchers had hoped.
This article will review 2 other strategies under investigation: (1) structured treatment interruption aims to increase the immunologic control over viral replication, while giving patients a break in the long and difficult journey through antiretroviral therapy, and (2) treatment intensification aims to avoid premature failure of an insufficiently potent antiretroviral regimen.
Structured Treatment Interruption
Structured Treatment Interruptions (STIs) – drug holidays initiated with the clinician’s guidance – are being studied for use in patients who have experienced virologic failure on multiple regimens and in patients who have achieved virologic suppression on their current regimen.
STI in Patients with Virologic Failure On Multiple Regimens
STI strategy was originally aimed at patients who had experienced virologic failure on multiple regimens and were left without therapeutic options. The theory was that cessation of treatment might result in reversion of resistant virus to wild type, thus allowing for the recycling of antiretroviral drugs. Unfortunately, neither V. Miller (the first scientist to propose and test this approach) nor S. Deeks has been able to confirm the encouraging preliminary results Miller obtained in 1999. STI frequently results in reversion to the wild-type virus in plasma, but the same does not occur in cell-associated virus. In addition, a marked increase in viral fitness occurs during therapy interruption. Finally, STI is associated with heavy decreases in patients’ CD4-cell counts, which may be associated with a high incidence of new adverse clinical events. Taken together, these data cast serious doubt on STI in patients with advanced HIV disease.
However, although no controlled clinical data exist, the majority of participants in pilot studies have experienced favourable changes in their HIV drug-resistance during treatment interruption. Resistance assays performed on peripheral blood samples in patients still taking their failing drug regimens showed a number of resistance-associated mutations in HIV reverse transcriptase and protease genes. After approximately 1 to 2 months off therapy, these genetic changes were less detectable as measured by both genotypic and phenotypic assays.
STI in Fully Suppressed Patients
A very different philosophy underlies the use of STI in patients who have achieved complete and persistent viral suppression. There is evidence that specific and effective cellular immune responses to HIV occur in infected subjects. As a result, researchers have explored alternative approaches to HIV therapy aimed at enhancing the host’s immune response. Encouraging results have been obtained with interleukin-2 and with HIV-derived immunogen therapies, and studies of other immunologic intervention strategies are ongoing. Effective control of HIV by a specific CD4-cell response in long-term nonprogressors suggests that enhancing the immune response may lead to a stable equilibrium between virus and host.
In this context, STI represents a promising approach to immune boosting. The hope is that short treatment interruptions will augment the length and strength of host immune responses to HIV. However, in the few completed uncontrolled studies of treatment interruptions, the majority of patients experienced a rapid viral-load rebound and a rapid decline in CD4-cell counts. As a result, the potential immunologic effectiveness of STI remains a matter of controversy to be clarified by controlled clinical trials.
The first uncontrolled trial of STI was essentially a “proof of concept,” demonstrating that HIV eradication is an unfeasible and unrealistic objective. However, this study also provided some basic data concerning CD4-cell and viral dynamics during therapy interruption and reinstitution. In further reports, Davey and colleagues showed that the viral-load rebound seen during therapy interruption was associated with a re-expansion of latently infected memory CD4 cells.
At approximately the same time, 2 isolated reports in the scientific literature (one of which described the much-discussed “Berlin patient”) seemed to suggest that interrupting antiretroviral treatment in patients who had achieved “undetectable” viral loads might result in exposing immune cells to “forgot-ten” viral antigens, thereby activating specific CD4-lymphoproliferative and CD8-cytotoxic responses. In theory, this sort of endogenous vaccination might even be more effective than therapeutic vaccines based on exogenous viral sequences. Several small clinical trials were then conducted in various countries to verify the effects of STI of limited duration (usually no more than 4 weeks) in HIV-positive patients with fully and persistently suppressed viral replication. Despite the many differences in study design, some general conclusions can be drawn from these trials:
1. STIs, at least in the approaches tested to date, appear to be safe; the HIV during viral rebound shows a conserved drug susceptibility profile, and reinstitution of treatment promptly results in a reduction in viral load and an increase in CD4-cell count.
2.An increase in HIV-specific CD4-lymphoproliferative activity and some favourable effects on the breadth and magnitude of CTL response has been observed in varying proportions of patients across these studies. However, it remains unclear how long these STI-induced immune responses persist.
3. A few cases have shown a reduced virologic set point after repeated STIs, although we do not know if this reduction is due to treatment interruption or if it is just a casual event.
Giving Patients a Break
STI may have a third and larger role in antiretroviral therapy, beyond battling resistance in patients with virologic failure or boosting immune responses in patients with virologic success. For as long as there has been antiretroviral therapy for HIV, patients have interrupted it. Treatment interruptions have occurred because of drug toxicity, intercurrent illness, boredom with treatment, chaotic life style, uncertainty over therapeutic advice, and many other reasons. In the early years of antiretroviral therapy, forced treatment interruptions were commonplace when patients experienced toxic reactions – because no new drugs were available. Now that there are more licensed antiretroviral agents, patients usually can change from regimen to regimen without needing to stop treatment, at least initially.
Many patients still wish to stop therapy temporarily (or permanently), for a particular social or medical reason – or for no stated reason whatsoever – and unplanned treatment interruptions are commonplace. While some patients can discuss treatment interruption with their physicians, others find this difficult, and many months may pass before their clinicians discover that treatment has ceased. Short unplanned treatment interruptions occur when pills are left at home, patients become forgetful, or life just becomes too complex.
We know that antiretroviral treatment has long-term toxicity and low tolerability and that it decreases quality of life for asymptomatic patients. Furthermore, clinical practice tells us that (1) at any given time, one third of patients on potent antiretroviral treatment have a viral load of more than 20,000 copies/ml, (2) unplanned treatment interruptions are common, and (3) virologic and immunologic failure and drug resistance occur at significant rates. The question becomes: Can we expect to keep people on antiretroviral therapy indefinitely, or should we try to give patients a break under certain circumstances, setting our sights on the larger goal of increasing their overall exposure to antiretroviral treatment? Interest is rising in using STI (also known in this context as “intermittent” or “pulse” therapy) to give patients just such a break from antiretroviral therapy’s associated toxicities and adherence burdens, with the ultimate goal of prolonging the beneficial effects of treatment. In addition, this strategy could have an unprecedented, positive effect on the treatment of HIV in resource-poor settings, from urban America to the developing world.
However, many questions still need to be answered in controlled trials before STI can be adopted in clinical practice:
- Could initiation of STI result in increased drug resistance because of the differential half life of antiretroviral drugs?
- What effect would repeated reinitiation of therapy have on early treatment side effects, such as rash or hypersensitivity, seen with some antiretroviral drugs?
- Will the response to therapy reinstitution be reproducible over an extended time period encompassing several treatment interruptions?
- What are the long-term effects on viral load and CD4-cell count?
- What are the long-term effects on genotypic and phenotypic resistance?
- Is STI strategy comparable, superior, or inferior to therapeutic vaccination?
- Will the response to STI be the same in PI-naive and PI-experienced patients?
- What happens during STIs in the various HIV reservoirs, such as the lymph nodes and semen?
“The current paradigm of HIV treatment is that it must be continuous throughout the patient’s life,” according to Mike Youle, the researcher who first proposed testing STI in clinical trials. “The assumption – based on our present knowledge of HIV viral dynamics and antiretroviral therapy – is that cessation of treatment would soon lead to reversion to pretreatment clinical status and be detrimental to the patient. However, the first studies of interrupting HIV treatment appear to challenge this assumption and offer evidence that the virus is more forgiving than we had previously believed.”
STI may have distinct attractions for patients and healthcare providers, offering the potential for reduced toxicity, improved tolerance, increased adherence, and lower cost. The ability to reuse drugs that had ceased to be effective and to reduce the overall exposure to antiretroviral agents is invaluable, especially given the limited number of available drugs. We must now eagerly await the results of controlled clinical trials. In the meantime, however, the use of STI outside of the clinical research setting must be discouraged.
Treatment Intensification
Despite the apparently wide array of available antiretroviral agents and the high number of theoretical combinations, cross-resistance among drugs of the same class limits therapeutic options. Thus, all efforts should be directed toward prolonging the effect of the initial antiretroviral treatment. Indeed, many controlled studies have proven that the nadir of a patient’s initial response correlates well with the durability of his or her response, and that achieving an early sustained response (by week 4 or 8 of therapy) is predictive of subsequent virologic suppression. Viral-load levels should therefore decrease by a minimum of 1.5 to 2.0 log over the first 4 weeks of therapy, and failure to achieve the target level of less than 50 copies/ml by 16 to 24 weeks should raise concern about patient adherence, drug absorption, or drug-resistant virus – although in patients with higher viral loads (e.g., more than 100,000 copies/ml) maximal viral suppression may take longer.
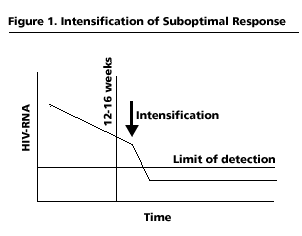
Figure 1
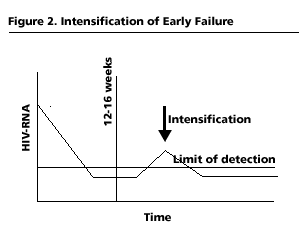
Figure 2
Intensification strategy represents an experimental approach to antiretroviral therapy designed to prolong the life of the initial regimen. Theoretically, it has applications in 2 different situations: (1) failure to establish initial control of virus replication and (2) “early” treatment failure after initial complete virologic response. (See Figures 1 and 2.) In persons with a low but detectable viral load following 12 to 16 weeks of potent antiretroviral therapy or in persons with early viral-load rebound after full suppression, the addition of a new drug could be an alternative to a complete regimen change, after other causes of treatment failure (notably incomplete adherence or drug resistance) are ruled out. One of the main goals of treatment intensification is the preservation of therapeutic options. Thus, drugs that can be used for intensification include agents from classes already represented in the regimen (preserving other classes in the event that intensification proves unsuccessful) or agents with high genetic barriers to resistance.
There are serious potential drawbacks to treatment intensification. Patient adherence is notoriously difficult to assess. Genotypic and phenotypic resistance testing, although promising, are still new features in clinical practice, and drug resistance can be difficult to rule out. In fact, given the thin line between intensification and incremental therapy, intensification may promote further drug resistance if it is already developing. Moreover, intensification may aggravate adherence problems and may intensify the level of adverse pharmacokinetic interactions. Further controlled studies of treatment intensification are certainly needed before this strategy can be used in standard clinical practice.
Conclusion
Over the past few years, HIV/AIDS – at least in developed nations – has been transformed into a chronic, treatable illness. Patients and clinicians are now in need of new therapeutic strategies to maximize the beneficial effects of the available antiretroviral drugs. STI and, to a lesser extent, treatment intensification represent promising new approaches to HIV care and merit intensive study.
Source: http://www.accnewsletter.org
Comment
The SSIT study, presented at Geneva, but not listed was, overall, negative although the presentation involved clutching at ‘encouraging’ individual patient data. One patient developed documented 3TC NFV resistance, another ‘failed’ to reinduce full suppression over 8 weeks. CD4 cell counts stayed flat, instead of the rise we might expect over one year appropriate therapy and mean VL rebound did not change.
Interruption schedules and timing remains poorly defined and may have a crucial role to play in any likely successes with this approach. Further research is required to more clearly define the role of controlled bursts of viraemia and possible dangers.
References:
- Altfield M et al. Increase in breadth and frequency of CTL responses after structured therapy interruptions in individuals treated with HAART during acute HIV-1 infection. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Carcelain G, et al. Intermittent interruptions of antiretroviral therapy in chronically HIV-infected patients do not induce immune control of HIV. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Carpenter C J et al. Antiretroviral therapy in adults. JAMA 2000; 283:381-390.
- Carr A et al. Diagnosis, prediction and natural course of HIV-1 protease inhibitor associated lipodystrophy, hyperlipidaemia and diabetes mellitus. Lancet 1999 ; 353:2093-2099.
- Davey R et al. The NoHRT trial: a prospective study of cessation of HAART in HIV-infected patients after prolonged viral suppression. 39th ICAAC, San Francisco 1999
- Deeks SG et al. Virologic and immunologic evaluation of structured treatment interruptions (STI) in patients experiencing long-term virologic failure. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Devereux H et al. Rapid decline in detectability of HIV-1 drug resistance mutations after stopping therapy. AIDS 1999 ; 13:F123-F127.
- Finzi D et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1. Nat Med 1999 ; 5:512-517.
- Garcia F et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS 1999 ; 13:F79-F86.
- Garcia F et al. Structured cyclic antiretroviral therapy interruption (STI) in chronic infection may induce immune responses against HIV-1 antigens associated with spontaneous drop in viral load. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Hatano H et al. Plasma viral loads approximate pre-HAART levels after discontinuation of HAART. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Jin X et al. Discontinuation of HAART after a course of therapeutic vaccination with ALVAC1452 and rgp160 may be associated with delayed viral rebound kinetics. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Lori F et al. Control of viremia after treatment interruption. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Neumann A et al. HIV-rebound during interruption of highly antiretroviral therapy has no deleterious effect on reinitiated treatment. AIDS 1999 ; 13:677-683.
- Pallela F et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998 ; 338:853-860.
- Papasavvas E et al. Boosting of HIV-1-specific cellular immune responses in chronically infected persons after treatment interruption. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Ruiz L et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. 7th European Conference on Clinical Aspects and Treatment of HIV Infection, Lisbon 1999
- Ruiz L et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Sherer R et al. No detectable HIV RNA in thirteen individuals months after stopping antiretroviral therapy. 7th Conference on Retroviruses & Opportunistic Infections, San Francisco 2000
- Youle M et al. Surrogate marker responses to multidrug combinations comprising hydroxyurea, efavirenz, double protease inhibitors and nucleoside analogues in protease inhibitor failures. 6th Conference on Retroviruses & Opportunistic Infections, Chicago 1999
- Youle M. Is interrupting HIV Therapy always harmful? J Antimicrob Chemotherapy 2000 ; 415:1-2.

