Dolutegravir/3TC dual ART is as effective at lowest viral load cut-off as triple therapy in GEMINI studies
28 March 2019. Related: Conference reports, Antiretrovirals, Treatment strategies, CROI 26 (Retrovirus) 2019.
 Simon Collins, HIV i-Base
Simon Collins, HIV i-Base
The phase 3 GEMINI 1 and 2 studies showed that dual therapy (2D) with dolutegravir/3TC was non-inferior to triple therapy (3D) with dolutegravir plus TDF/FTC, based on viral suppression at 48 weeks.
A new analysis, presented as a poster at CROI 2019, now also shows no differences between the two arms at viral load <40 copies/mL.
The analysis was based on participants with viral load <40 copies/mL having either a target detected (TD) or target not detected (TND) result.
At the primary 48 week endpoint and at all earlier time points there were no significant differences between the 2D vs 3D arms: 77% vs 73% (adjusted difference: 3.8%, 95% CI –0.6%, 8.2%).
Proportions were similar at all other time points: 34% vs 32% (week 4), 52% vs 49% (week 8), 60% vs 57% (week 12), 59% vs 56% (week 16), 65% vs 63% (weeks 24) and 65% vs 68% (week 36), in the 2D vs 3D arms respectively.
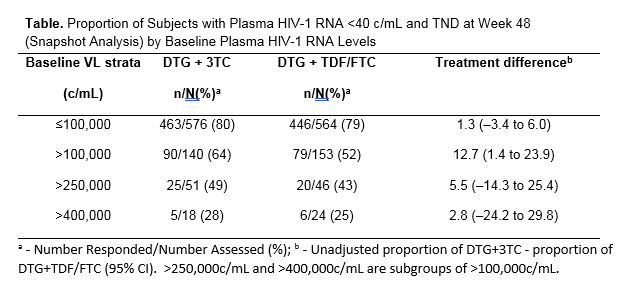
By baseline viral load, there was also no differences between arms in participants starting <100,000 copies/mL but a numerical advantage for the 2D arm when baseline viral load was >100,000 copies/mL (64% vs 52% (difference +12.7% (95%CI: 1.4 to 23.9). See Table 1.
Time to TND was the same for both arms: 29 days with baseline VL <100,000 and 57 days when >100,000 copies/mL.
Reference
Underwood M et al. HIV replication at <40 c/mL for DTG+3TC vs DTG+TDF/FTC in the GEMINI 1 & 2 studies. CROI, 4-7 March 2019, Poster abstract 490.
http://www.croiconference.org/sessions/hiv-replication (abstract)

