Impressive HIV pipeline at CROI 2017
26 June 2017. Related: Conference reports, Coinfections and complications, CROI 24 (Retrovirus) 2017.
CROI 2017 was notable for perhaps presenting the strongest collection of studies on the HIV treatment pipeline for years. And while current ART is safe and effective these studies showed ways it could become better still.
There are plenty of good aims for better drugs.
Treatment could involve smaller pills, less frequent dosing, long-acting formulations (weekly, monthly, yearly), with lower doses, fewer side effects and drug interactions, stronger resistance profiles and they could be cheaper and more accessible.
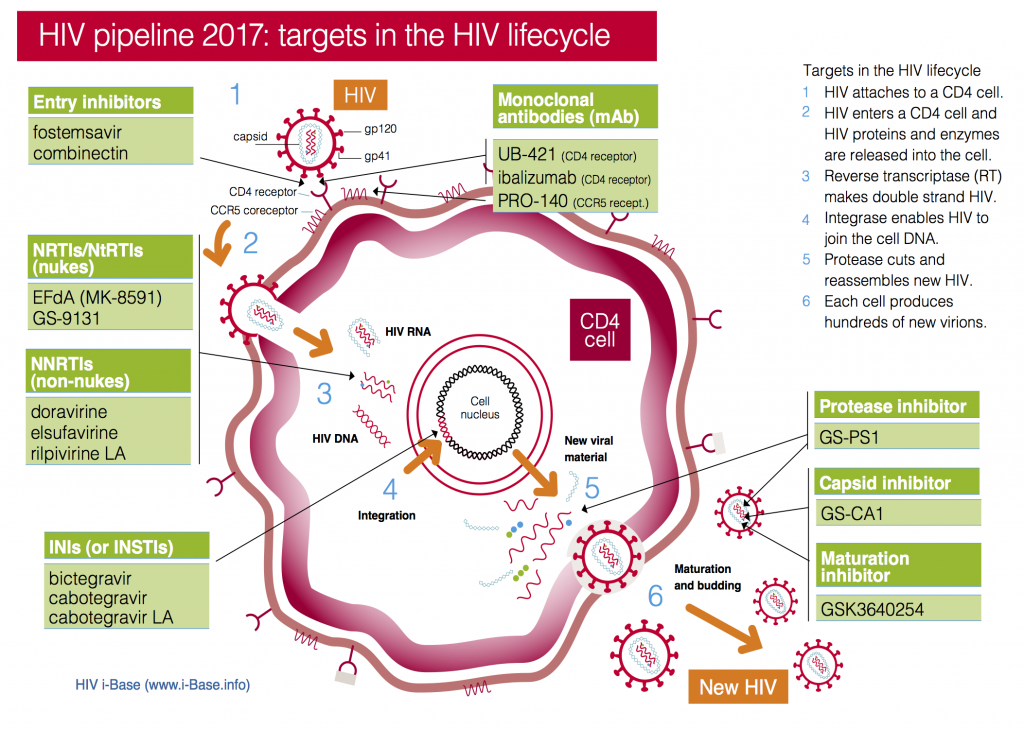
Drug targets for HIV pipeline compounds 2017
CROI included research into new drugs in current classes: nukes, NNRTIs, PIs and integrase inhibitors – and compounds with new targets and mechanisms of action – including a capsid inhibitor and a range of different monoclonal antibodies.
The brief summaries below Table 1 link to the abstracts, posters and webcasts from the meeting.
| Compound | Comment | Phase | CROI 2017 Refs |
|---|---|---|---|
| NRTI’s | |||
| EFdA (MK-8591) | Highly potent, low dose, active against NRTI resistance. Long half-life, potential as oral (weekly dose) and implant (annual implant for PrEP). | Phase 1 | CROI 2017: Abs 435 and 440. |
| GS-9131 prodrug of GS-9141 | Active against NRTI resistance. Synergy reported with AZT, FTC, abacavir, efavirenz, bictegravir, dolutegravir and lopinavir, and additive activity with TFV and TAF. Will be coformulated with other Gilead drugs.Currently difficult to synthesise in bulk. | Pre-clinical | CROI 2017: Abs 436. |
| NNRTI’s | |||
| doravirine | Active against first generation NNRTI resistance. Non-inferior to efavirenz. Generic FDC with TDF/3TC in phase 3 studies. | Phase 3 | CROI 2017: Abs 45LB. CROI 2016: Abs 470. |
| Elsulfavirine, prodrug of VM-1500A | Only being developed for use in low and middle income countries. Similar activity to efavirenz in phase 2 Russian study, | Phase 2 | CROI 2017: Abs 452LB. |
| Integrase inhibitors (INSTI’s) | |||
| dolutegravir | Already approved integrase inhibitor but now in a new two-drug coformulation with the NRTI rilpivirine. | FDC with RPV now submitted. | CROI 2017: Abs 44LB. |
| cabotegravir | Oral formulation integrase inhibitor. | Phase 2. | CROI 2017: Abs 442. |
| cabotegravir LA | Injection with very long half-life – detectable after more than one year following single injection. Research as both treatment and prevention. | Phase 3 (for both ART and PrEP) | CROI 2017: Abs 84 and 439. |
| bictegravir | Once-daily, unboosted, low-dose with completed phase 3 studies and coformulation as part of an FDC with FTC/TAF. | Submitted to FDA. | CROI 2017: Abs 41. |
| GS-9695 and GS-9822 | Non-catalytic integrase inhibitors no longer being studied due to renal toxicity. | Stopped. | CROI 2017: Abs 434. |
| Protease inhibitors | |||
| GS-PI1 | New QD unboosted PI, high potency, long half-life, potential in FDC single table regimen (Gilead). | Pre-clinical | CROI 2017: Abs 433. |
| Capsid inhibitors | |||
| GS-CA1 | Early stage for new class with activity at multiple stages of viral lifecycle. Sub-cutaneous injection with monthly or less frequent dosing. | Pre-clinical | CROI 2017: Abs 38. |
| Monoclonal antibodies (mAbs) | |||
| PRO 140(CCR5 target) | Once-weekly (350 mg) sub-cutaneous injection with potential to maintain viral suppression for more than two years after stopping ART. Also, with ART against multiclass resistance. | Phase 3 | CROI 2017: Abs 437. |
| ibalizumab(CD4 binding site) | Intravenous infusion (800 mg every two weeks) being studied in addition to optimised ART in single arm study in people with multiclass HIV drug resistance. Previously called TNX-355. | Phase 3 | CROI 2017: Abs 438 and 449 LB. |
| VRC01(CD4 binding site) | Intravenous infusion (40 mg/kg) being studied with ART for effect on reservoir and in cure research and as PrEP (2 large phase 3 studies are ongoing). Sub-cutaneous dosing of infants to prevent transmission at birth or from breastfeeding. | Ph 1 (infants), ph 2 (cure-related and adult PrEP) | CROI 2017: Abs 330 LB and 760. |
| UB-421 | Infusion dosed either weekly or every two weeks as alternative to ART during treatment interruption. | Phase 2 | CROI 2017: Abs 450 LB. |
NRTIs/NtRTIs
NRTIs are the oldest class of drugs but are still the backbone of most ART combinations. Two new compounds in the pipeline are of particular interest.
EFdA (MK-8591)
EFdA is an NRTI now in development by Merck (development name MK-8591) that is notable for high potency (10 mg oral dose), long plasma half-life that allows once-weekly oral dosing, a slow-release removable implant that might only require annual dosing and ongoing studies looking at use for both treatment and prevention.
A poster at CROI 2017 reported that MK-8591 also had even better activity in vitro against HIV-2 compared to six HIV-1 isolates (including HIV-1 subtypes A, B, C and D and group O). This includes being fully active against NRTI mutations K65R and Q151M (although the M184V variant conferred 10-fold resistance). [1]
Another poster reported that EFdA reaches good drug levels in vaginal and rectal tissue – supporting further PrEP studies. [2]
GS-9131 – NRTI with activity against NRTI resistance
GS-9131 is a prodrug of GS-9148 about which early animal and in vitro drug resistance studies presented at CROI 2006. [3]
Other published studies highlight the potential for low risk of toxicity in animal studies and retains in vitro phenotypic sensitivity to broad NRTI resistance including mutations at K65R, L74V and M184V and multiple TAMS. [4]
The poster at CROI 2017 confirmed results from previously published studies into the activity against common NRTI mutations. [5]
The compound has good potency (EC50 = 25-200 nM) with activity against HIV-1 subtypes A, B, C, D, E, F, group O and N (EC50 0.29-113 nM), also against HIV-2. Synergistic activity was reported for GS-9131 in combination with AZT, FTC, abacavir, efavirenz, bictegravir, dolutegravir and lopinavir, and additive activity with TFV and TAF.
Currently, GS-9131 is not easy to synthesise and it will need to overcome manufacturing challenges to become easier and cheaper to make.
NNRTIs
Doravirine
Doravirine is a once-daily NNRTI from Merck that can be taken with or without food and few drug-interactions and that retains activity against common first generation NNRTI mutations (K103N, Y181C, G190A and E138K).
In a late breaker oral presentation at CROI 2017, doravirine was non-inferior to boosted darunavir in a phase 3 study, showing similar viral suppression and low rates of side effects at the 48-week primary endpoint. [6]
Last year at CROI, results from a phase 2b study reported non-inferiority compared to efavirenz. [7] A fixed dose combination of doravirine/TDF/3TC (using generic NRTIs) is already in phase 3 studies and a long-acting formulation is in development. [8, 9]
Two posters were also presented this yearon (i) increased doravirine levels from a drug interaction with ritonavir [10] and (ii) on the ability to use doravirine in severe renal impairment (eGFR < 30 mL/min/1.73 m2) without dose adjustment [11].
Elsulfavirine, prodrug of VM-1500A
Elsulfavirine is an NNRTI being developed by Viriom for registration in low-income countries.
Results from 48-week were presented at CROI 2017 from a randomised, double-blind phase 2b study conducted in Russia in 120 treatment naive participants. Elsulfavirine 20 mg was compared to efavirenz 600 mg, each with tenofovir-DF/FTC background NRTIs. [12]
The elsulfavirine arm reported similar viral suppression to <50 copies/mL (81% vs 73%), including those with baseline viral load >100,000 copies/mL (78% vs 62%), with fewer CNS side effects (32% vs 62%).
A long-acting injectable formulation is being used in ongoing studies for treatment and PrEP and results will be presented at IAS 2017 in Paris this summer.
Protease inhibitors
GS-PI1
GS-PI1 is a once-daily unboosted protease inhibitor with high potency and a long half-life, and in vitro sensitivity against some second-generation PI resistance, in pre-clinical development by Gilead.
An oral presentation at CROI 2017 reported a high barrier to resistance both after in vitro passaging and against multiple resistance complexes from multiple PI-resistant clinical isolates, and pharmacokinetic data from rat and dog studies. [13]
Integrase inhibitors
Dolutegravir/rilpivIRine dual therapy
Although already approved as an oral integrase inhibitor, results from the SWORD 1 and 2 studies were presented at CROI 2017 for use with oral rilpivirine as two-drug maintenance therapy. [14]
Combined results in 1024 participants with undetectable viral load for >12 months and no history of treatment failure that were randomised to switch to either dolutegravir/rilpivirine or continue current ART. Switching to dual arm was non-inferior to continuing ART in all analysis at week-48 including suppression to <50 copies/mL (ITT: 95% vs. 95%; difference: –0.4%; 95%CI: –3.1% to +2.3%).
Based on these results coformulated fixed dose combination with the NNRTI rilpivirine has already been submitted to the US FDA for approval as a two-drug maintenance therapy. [15]
Cabotegravir
The original oral formulation of this integrase inhibitor was initially used for run-in phase for studies using long-acting injections. Similar to the dolutegravir study above, results were presented at CROI 2017 using oral cabotegravir together with oral rilpivirine as a two-drug maintenance therapy. [16]
This phase 2b study presented 144 week results on 243 patients who started therapy with triple therapy (dose ranging CAB or efavirenz, plus background TDF/FTC NRTIs, and who switched to oral CAB plus rilpivirine maintenance therapy at week 24 if viral load was undetectable.
Of 181 participants randomised to CAB, 160 began the dual maintenance phase and 138 entered a further open label phase at week 96.
Although virologic suppression was generally good and tolerability included no cabotegravir-related discontinuations for side effects, five patients developed resistance to one or both drug during the study.
During the maintenance and open label phases, 7 (4%) reported drug-related adverse events (AEs) ≥ Grade 2. Serious AEs occurred in 15 (9%) participants in the CAB group (none drug related) and 4 (3%) withdrew due to AEs.
Cabotegravir-LA
Cabotegravir LA is a long-acting integrase inhibitor in late-stage phase 3 studies using two-monthly dose intramuscular injections both for HIV treatment and PrEP prevention.
The extremely long half-life (detectable pharmacokinetic tail more than year after a single injection) brings new concerns linked to stopping treatment both for use in ART and as PrEP
With ART, substituting a new drug is easy given treatment interruptions are now rare, but the risk of drug resistance developing in case of PrEP failure is more than a theoretical concern as this has been reported with current oral PrEP when started in very early infection (ie before HIV can even be detected).
An animal study at CROI showed similar concerns are important for cabotegravir. Six monkeys were given cabotegravir shortly after being infected with SIV and 4/6 developed integrase resistance. The concern from integrase resistance is that it might limit the option of integrase inhibitor treatment in people who become positive. [17]
Although the current cabotegravir formulation is well tolerated in studies, it requires a relatively large volume (two injections) into muscle. Research into easier formulations that has absorption and HIV activity at lower doses was presented in a poster. [18]
Bictegravir
Bictegravir (formerly GS-9883) is a once-daily integrase inhibitor with a plasma half-life of 18 hours that has in vitro activity against many integrase-associated mutations. Bictegravir has high protein binding (99%) limiting penetration to CSF but does not require boosting or need to be taken with food.
Two oral presentations were presented at CROI 2017, most importantly results from a phase 2 study (also published in the Lancet) which showed non-inferior efficacy and tolerability compared to dolutegravir. [19, 20]
Drug interaction studies at the conference reported increased bictegravir AUC (61-74%) by CYP3A4 inhibitors (voriconazole and darunavir/c), with a greater increase (~4-fold) by potent dual inhibitors of UGT1A1 and CYP3A4 (atazanavir and atazanavir/c). Bictegravir AUC is reduced by 75% by rifampin, a potent CYP3A4/UGT1A1/P-gp inducer and by 38% with rifabutin. [21]
Phase 3 studies are completed using a fixed dose combination coformulated with FTC/TAF, with comparator drugs that include dolutegravir and darunavir in treatment-naive studies and plus various switch studies for people already on treatment. This FDC uses an improved formulation of bictegravir that only requires a 50 mg dose.
On 12 June 2017, the FDC of BIC/FTC/TAF was submitted to the US FDA for approval. [22]
GS-9695 and GS-9822
GS-9695 is a non-catalytic integrase inhibitor (NCINI) that binds to a conserved pocket on the enzyme that is also targeted by LEDGF. The compound (previously developed by Boehringer Ingelheim as BI-224436) has potential for low dose, once-daily dosing, unboosted, with high barrier to drug resistance. A follow-on compound GS-9822 strengthened the resistance profile.
However, a poster at CROI 2017 reporting unpredictable kidney/urothelial toxicty in monkeys has led to discontinuation of development of this compound. [23]
Capsid inhibitors
GS-CA1
First data was presented on GS-CA1, the first compound in a new class of HIV capsid inhibitors, with a formulation that can be used for slow-release injections. [24]
Capsid is the cone-shaped structural core within the virion that protects HIV RNA and related enzymes. As part of a dynamic process, the capsid protein (p24) first breaks down to release viral contents into the CD4 cell to enable reverse transcription and also needs to reassemble inside new virions as part of the maturation process the end of the lifecycle.
GS-CA1 acts both the early and late stages by binding at a site that blocks both disassembly and assembly leading to defective new virions that are non-infectious.
The compounds is potent with EC50 in target cells of 60 to 140 pM (compared to 1000 to 19000 for efavirenz, dolutegravir and atazanavir) with activity against drug resistance to current HIV classes. Although population sequencing showed the binding site to be highly conserved, capsid resistance can be generated from in vitro serial passaging.
The investigational compound is currently developed as a subcutaneous injection that in rat studies maintained plasma concentrations nine times above the protein adjusted EC95 ten weeks after a single injection. This suggests that monthly or longer dosing intervals in humans.
Monoclonal antibodies (mAb)
CROI 2017 included important new results for several broadly neutralising monoclonal antibodies (mAbs) given by infusion or injection, some of which have been the focus of research for many years. These compounds have potential to be used in very different settings, with use as treatment, as a cure strategy and as prevention.
This is a rapidly developing new class with multiple target sites and numerous compounds, with the expectation that combination mAb therapy will be required to prevent viral escape. [25]
PRO 140
PRO 140 is a humanised IgG4 antibody that blocks HIV entry by binding to CCR5 but which is active against maraviroc-resistant virus. PRO 140 has been in development for more than ten years, that paradoxically has designated “fast-track” status, with potential for activity against multiclass HIV drug resistance.
The current study used weekly dosing of 350 mg self-administered sub-cutaneous injections of PRO 140 monotherapy as a switch strategy in participants on stable oral ART with undetectable viral load, who interrupted ART. This was initially a 12-week study with a three-year extension follow-up for people who maintain viral suppression. [26]
Of 41 participants from the first phase, 16 joined the extension phase. Of these, 1/16 withdrew consent, 5/16 had subsequent viral rebound (two consecutive results >400 copies/mL) and 10/16 have maintained viral suppression with follow-up for longer than two years. Of these, 7/10 had undetectable viral load <1 copy/mL, with others at 4, 10 and 19 copies/mL.
No serious side effects or related discontinuations were reported, including low inject site reactions.
Ongoing phase 3 studies include (i) monotherapy switch study in 300 participants with viral suppression >48 weeks on ART, and (ii) in addition to ART as part of salvage combination in 30 participants with multidrug resistance to other classes.
Ibalizumab
Ibalizumab is another humanised IgG4 antibody in phase 3 studies that blocks HIV entry and that has been a potential HIV pipeline compound for well-over ten years. Ibalizumab blocks the CD4 receptor and is active against both CCR5- and CXCR4-tropic HIV.
Two studies were presented at CROI 2017. One was a single-arm, 24-week, open label study in 40 extensively treatment-experienced participants adding ibalizumab (800 mg IV every two weeks) to background ART (optimised from day 14). [27, 28]
Mean baseline viral load was 5.0 log copies/mL with 18% > 5 log copies/mL. Mean CD4 was 150 cells/mm3 with 17 people < 50 cells/mm3 and 10 people with CD4 between 50 to 200 cells/mm3. More than half the participants had resistance to three classes, one-third to four classes and 40% used another investigational drug (the gp-120 attachment inhibitor fostemsavir).
One week (at day 7) after the initial loading dose (2000 mg IV) added to current failing therapy, viral load dropped by > 0.5 log copies/mL in 83% of participants and > 1.0 log in 60%.
At week 24, mean viral load decrease from baseline was -1.6 log copies/mL, with 55% and 48% having reductions >1 log and >2 log respectively. Viral load was undetectable (<50 copies/mL) in 43% of participants. Baseline CD4 > 50 cells/mm3 was associated with greater viral load reductions.
There were 9/40 discontinuations, mostly (8/9) in patents with lowest CD4 (<50 cells/mm3). This included 4 deaths (liver failure, KS, end-stage AIDS and lymphoma), all in the low CD4 group. Three people withdrew consent and two were lost to follow-up.
Side effects (n=17) were mostly mild or moderate, but included one case of IRIS.
The second study was a phase 1/2 pharmacokinetic study using an 800 mg intramuscular formulation of ibalizumab in eight HIV positive people not on ART.
Mean viral load and CD4 count at baseline was 55,000 copies/mL and 314 cells/mm3 respectively.
After the initial 3-day peak, drug levels were comparable to historical data using the IV formulation.
However, maximum viral load reductions (~1.2 log copies/mL) occurred by day 7, subsequently returning to baseline (likely due to resistance) compared to reductions of ~2 log/copies/mL at day 14 with the 2000 mg loading dose with the infusion formulation that was sustained for 24 weeks.
Tolerability was improved using the IM formulation.
VRC01
VRC01 is another broadly neutralising mAb that targets the CD4 binding site that can be given by infusion or sub-cutaneous injection and that is being studied for multiple potential uses.
One study at CROI reported no additional impact on reducing the latently infected viral reservoir from adding VRC01 to ART. [29] Other roles in cure research is ongoing [30].
Another study reported tentatively positive safety results from using a single injection in infants after birth to limit risk of mother-to-child transmission with a potential role of additional injections when breastfeeding is a risk. [31]
Two other large international dose-finding, placebo-controlled phase 2 VRC01 PrEP studies are already ongoing that allow the option for participants to also use open-label oral TDF/FTC PrEP. [32, 33]
UB-421
UB-421 is a third mAb that targets CD4 binding that had data presented at CROI 2017 from a phase 2 study in 29 virally suppressed participants on ART who used UB-421 monotherapy during an 8-week treatment interruption. [34]
UB-421 was given by infusion either 10 mg/kg weekly or 25 mg/kg every two-weeks. Although there were no cases of viral rebound during the monotherapy phase, viral load did rebound at 35 to 62 days after the last UB-421 dose in five participants who delayed restarting ART.
All five later restarted ART and viral load became undetectable.
References:
Unless stated otherwise, references are to the Programme and Abstracts of the 24th Conference on Retroviruses and Opportunistic Infections (CROI 2017), 13-16 February 2017, Seattle, Washington.
- Wu V et al. Antiviral activity of EFdA against NRTI-sensitive and resistant strains of HIV-2. CROI 2017, 13-16 February, Seattle, Washington. Poster abstract 440.
http://www.croiconference.org/sessions/antiviral-activity-efda-against-nrti-sensitive-and-resistant-strains-hiv-2 - Grobbler J et al. MK-8591 concentrations at sites of HIV transmission and replication. CROI 2017, 13-16 February, Seattle, Washington. Poster abstract 435.
http://www.croiconference.org/sessions/mk-8591-concentrations-sites-hiv-transmission-and-replication - Cihlar T et al. GS9148: A novel nucleotide active against HIV-1 variants with drug-resistance mutations in reverse transcriptase. 13th Conference on Retroviruses and Opportunistic Infections, 5-8 February 2006, Denver. Oral abstract 45. (No longer available online).
- Cihlar T et al. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of HIV Type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob Agents Chemother. 2008 Feb; 52(2): 655-665. Published online 2007 Dec 3. doi: 10.1128/AAC.01215-07.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2224772 - White KL et al. GS-9131 is a novel NRTI with activity against NRTI-resistant HIV-1. CROI 2017, 13-16 February, Seattle. Poster abstract 436.
http://www.croiconference.org/sessions/gs-9131-novel-nrti-activity-against-nrti-resistant-hiv-1 - Molina J-M et al. Doravirine is non-inferior to darunavir/r in phase 3 treatment-naive trial at week 48. CROI 2017, 13-16 February, Seattle. Late breaker oral abstract 45LB.
http://www.croiconference.org/sessions/doravirine-non-inferior-darunavirr-phase-3-treatment-naïve-trial-week-48 - Gatell JM et al. Doravirine 100mg QD vs efavirenz +TDF/FTC in ART-naive HIV+ patients: week 48 results. 23rd CROI, 22 – 25 February 2016, Boston. Poster abstract 470.
http://www.croiconference.org/sessions/doravirine-100mg-qd-vs-efavirenz-tdfftc-art-naive-hiv-patients-week-48-results (Abstract)
http://www.croiconference.org/sites/default/files/posters-2016/470.pdf (PDF poster) - ClinicalTrials.gov [Internet]. Comparison of MK-1439A and ATRIPLATM in treatment-naive human immunodeficiency virus (HIV)-infected participants (MK-1439A-021). Identifier NCT02403674.
https://clinicaltrials.gov/ct2/show/NCT02403674 - ClinicalTrials.gov [Internet]. A rapid pharmacokinetic trial of the bioavailability of four MK-1439 nano formulations in healthy adults. Identifier NCT02549040.
https://clinicaltrials.gov/ct2/show/NCT02549040 - Khalilieh S et al Multiple-dose treatment with ritonavir increases the exposure of doravirine. CROI 2017, 13-16 February, Seattle. Poster abstract 412.
http://www.croiconference.org/sessions/multiple-dose-treatment-ritonavir-increases-exposure-doravirine - Ankrom
W et al. Effect of severe renal impairment on doravirine pharmacokinetics. CROI 2017, 13-16 February, Seattle. Poster abstract 430.
http://www.croiconference.org/sessions/effect-severe-renal-impairment-doravirine-pharmacokinetics - Murphy R et al. Elsulfavirine as compared to efavirenz in combination with TDF/FTC: 48-week study. CROI 2017, 13-16 February, Seattle. Late breaker abstract 452LB.
http://www.croiconference.org/sessions/elsulfavirine-compared-efavirenz-combination-tdfftc-48-week-study - Link JO et al. Novel HIV PI with high resistance barrier and potential for unboosted QD oral dosing. CROI 2017, 13-16 February, Seattle. Late breaker abstract 433.
http://www.croiwebcasts.org/p/2017croi/croi33636 - Llibre JM et al. Phase III SWORD 1&2: Switch to DTG+RPV/ maintains virologic suppression through 48 wks. CROI 2017, 13-16 February, Seattle. Late breaker oral abstract 44LB.
http://www.croiconference.org/sessions/phase-iii-sword-12-switch-dtgrpv-maintains-virologic-suppression-through-48-wks - ViiV Healthcare press statement. ViiV Healthcare submits regulatory applications for the first HIV maintenance regimen comprising only two medicines. (01 June 2017).
https://www.viivhealthcare.com/media.aspx - Margolis DA et al. Long-term safety and efficacy of CAB and RPV as 2-drug oral maintenance therapy. CROI 2017, 13-16 February, Seattle. Poster abstract 442.
http://www.croiconference.org/sessions/long-term-safety-and-efficacy-cab-and-rpv-2-drug-oral-maintenance-therapy - Radzio J et al. Resistance emergence in macaques administered cabotegravir LA during acute infection. CROI 2017, 13-16 February, Seattle. Oral abstract 84.
http://www.croiconference.org/sessions/resistance-emergence-macaques-administered-cabotegravir-la-during-acute-infection - Tian Zhou T et al. A long-acting nanoformulated cabotegravir prodrug for improved antiretroviral therapy. CROI 2017, 13-16 February, Seattle. Poster abstract 439.
http://www.croiconference.org/sessions/long-acting-nanoformulated-cabotegravir-prodrug-improved-antiretroviral-therapy - Sax PE et al. Randomized trial of bictegravir or dolutegravir with FTC/TAF for initial HIV therapy. CROI 2017, 13-16 February, Seattle. Oral abstract 41.
http://www.croiconference.org/sessions/randomized-trial-bictegravir-or-dolutegravir-ftctaf-initial-hiv-therapy (abstract)
http://www.croiwebcasts.org/console/player/33378 (webcast) - Sax P et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. The Lancet HIV (14 February 2017). DOI: http://dx.doi.org/10.1016/S2352-3018(17)30016-4
http://thelancet.com/journals/lanhiv/article/PIIS2352-3018(17)30016-4/abstract - Zhang H et al. Clinical pharmacology of the HIV integrase strand transfer inhibitor bictegravir. CROI 2017, 13-16 February, Seattle. Oral abstract 40.
http://www.croiconference.org/sessions/clinical-pharmacology-hiv-integrase-strand-transfer-inhibitor-bictegravir (abstract)
http://www.croiwebcasts.org/console/player/33377 (webcast) - Gilead press statement. Gilead submits new drug application to U.S. food and drug administration for fixed-dose combination of bictegravir, emtricitabine and tenofovir alafenamide for HIV treatment. (12 June 2017).
http://www.gilead.com/news/press-releases - Mitchell ML et al. Novel non-catalytic site integrase inhibitor with improved resistance profile. CROI 2017, 13-16 February, Seattle. Poster abstract 434.
http://www.croiconference.org/sessions/novel-non-catalytic-site-integrase-inhibitor-improved-resistance-profile (abstract and PDF) - Tse WC et al. Discovery of novel potent HIV capsid inhibitors with long-acting potential. CROI 2017, 13-16 February, Seattle, Washington. Oral abstract 38.
http://www.croiconference.org/sessions/discovery-novel-potent-hiv-capsid-inhibitors-long-acting-potential (abstract)
http://www.croiwebcasts.org/p/2017croi/croi33375 (webcast) - Moore P et al. Neutralizing antibody development during HIV-1 infection
. CROI 2017, 13-16 February, Seattle. Oral abstract 142.
http://www.croiconference.org/sessions/neutralizing-antibody-development-during-hiv-1-infection (abstract and webcast) - Lalezari J et al. PRO140 single-agent maintenance therapy for HIV-1 Infection: a 2-year update. CROI 2017, 13-16 February, Seattle. Poster abstract 437.
http://www.croiconference.org/sessions/pro140-single-agent-maintenance-therapy-hiv-1-infection-2-year-update (abstract and poster)
http://www.croiwebcasts.org/p/2017croi/croi33640 (webcast) - Lewis S et al. Long-acting ibalizumab in patients with multi-drug resistant HIV-1: A 24-week study. CROI 2017, 13-16 February, Seattle. Poster abstract 449LB.
http://www.croiconference.org/sessions/long-acting-ibalizumab-patients-multi-drug-resistant-hiv-1-24-week-study (abstract and poster) - Lin H-H et al. Intramuscular ibalizumab: pharmacokinetics, safety, and efficacy vs IV administration. CROI 2017, 13-16 February, Seattle. Poster abstract 438.
http://www.croiconference.org/sessions/intramuscular-ibalizumab-pharmacokinetics-safety-and-efficacy-vs-iv-administration (abstract and poster) - Riddler S et al. VRC01 infusion has no effect on HIV-1 persistence in ART-suppressed chronic infection. CROI 2017, 13-16 February, Seattle. Late breaker poster 330LB.
http://www.croiconference.org/sessions/vrc01-infusion-has-no-effect-hiv-1-persistance-art-suppressed-chronic-infection (abstract and poster) - Schief WR et al. Immunogen design to induce HIV neutralizing antibodies. CROI 2017, 13-16 February, Seattle. Oral abstract 143.
http://www.croiconference.org/sessions/immunogen-design-induce-hiv-neutralizing-antibodies (abstract and webcast) - Cunningham CK et al. Safety & pharmacokinetics of the monoclonal antibody, VRC01, in HIV-exposed newborns. CROI 2017, 13-16 February, Seattle. Poster abstract 760.
http://www.croiconference.org/sessions/safety-pharmacokinetics-monoclonal-antibody-vrc01-hiv-exposed-newborns (abstract and poster) - ClinicalTrials.gov [Internet]. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection in women.
https://www.clinicaltrials.gov/ct2/show/NCT02568215 - ClinicalTrials.gov [Internet]. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection among men and transgender persons who have sex with men.
https://www.clinicaltrials.gov/ct2/show/NCT02716675 - Wang C-Y et al. A phase 2 open-label trial of antibody UB-421 monotherapy as a substitute for HAART. CROI 2017, 13-16 February, Seattle. Poster abstract 450 LB.
http://www.croiconference.org/sessions/phase-2-open-label-trial-antibody-ub-421-monotherapy-substitute-haart (abstract and poster)


