HIV pipeline 2020: new drugs in development – March 2020
12 March 2020. Related: Special reports, Pipeline report.
 Simon Collins, HIV i-Base
Simon Collins, HIV i-Base
Two versions of this report are available, both also as PDF files.
1. This full online version includes more information for each drug, with full references.
Download full version – PDF (500 Kb)
2. The “Pipeline-lite” version has a reduced summary for each drug and is included in the i-Base Fit For Purpose report.
Download reduced Pipeline-lite version – PDF (500 Kb)
Introduction: eight months since IAS 2019
This report is based on new developments over the last eight months since the pipeline report produced for the IAS conference in July 2019. [1]
This includes the move towards simplified ART and long-acting compounds in several drug classes that allows less frequent dosing than daily oral ARVs.
It also includes using bNAbs as a rescue treatment for people with multiclass HIV resistance. So while ibalizumab has already been approved with an orphan-drug designation for multidrug resistance, this potential is shared with dozens of other bNAbs and other new classes including long-acting capsid inhibitors.
It is notable that the companies developing new drugs for treatment and prevention are also investing in HIV cure.
The report also includes references to 30 studies at CROI 2020 that cover a wide range of pipeline compounds. They include islatravir, MK-8504 and 8583, bNAbs (including VRC01, PGT-121, GS-9722, 3BNC117, 10-1074, N6-LS), GS-6207 (capsid inhibitor), GSK3640254 (maturation inhibitor), elsulfavirine, combinectin, ABX464, BIT255 and GS-9131.
Figure 1: HIV pipeline 2020: targets in the HIV lifecycle
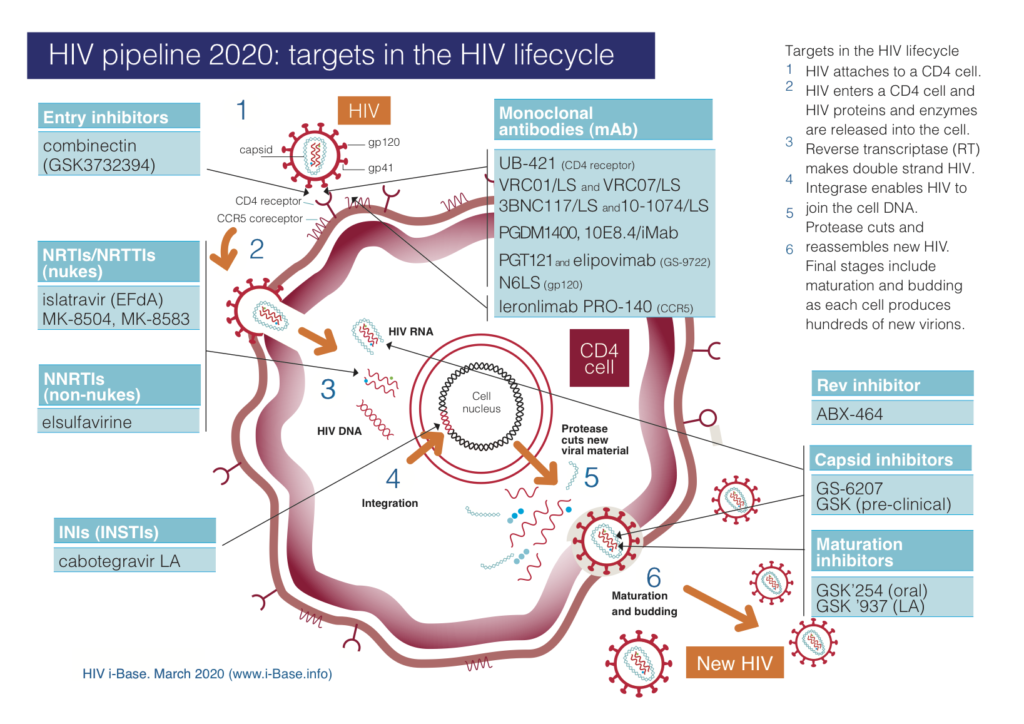
Key: INSTI: integrase strand transfer inhibitor; LA: long-acting; mAb: monoclonal antibody; NRTI: nucleoside/tide reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor.
Regulatory approvals and submissions (Table 1)
In September 2019, the HIV monoclonal antibody ibalizumab (Trogarzo) received approval in the EU as a treatment for multidrug HIV resistance (18 months after approval in the US). [2, 3] See Table 1.
And the gp-120 attachment inhibitor fostemsavir was also submitted to the FDA in December 2019 and to the EMA in January 2020 based on 96-week results from the BRIGHTE study in people with multidrug resistance. [4, 5, 6]
However, also in December, the regulatory application for the long-acting injections of cabotegravir and rilpivirine (Cabenuva) was not approved by the FDA within the fast-track timeline. [7] This was unexpected given good results from the phase 3 FLAIR and ATLAS studies. [8]
The explanation concerned manufacturing problems from scaling up to industrial production. Although the FDA complete response letter was not made publicly available, ViiV Healthcare say there were no safety and efficacy concerns and that all ongoing studies and expanded access programmes are continuing. The company is focused on resolving the manufacturing problems as soon as possible.
When cabotegravir/rilpivirine LA injections are approved, most health care settings will be looking at practical considerations that are needed before broad access. These include defining best population, how, where and who will be responsible for administering injections, complications from cold chain requirement for rilpivirine LA, whether alternative injection sites are possible (for example with sarcopenia), whether the oral lead-in period can be dropped for people who have difficulty swallowing pills and how to handle people who don’t return for repeat dosing. Some of these questions are already being addressed in ongoing studies. [9, 10, 11]
In October 2019, ViiV also submitted a new application to the FDA for a new indication for the dual combination of dolutegravir/lamivudine (Dovato). This will be as a switch option for people who are stable on a current combination using at least three drugs, based on 48-week results from the phase 3 TANGO study, presented at IAS 2019. [12, 13]
Dolutegravir/lamivudine was approved in the EU as first-line ART in July 2019 [14] and, also at IAS, extended follow-up to 96-weeks for the GEMINI 1 and 2 studies provided additional data supporting durability of this dual therapy in treatment naive participants. [15]
Updates from CROI 2020 include looking at the importance of other background drugs with ibalizubmab. [82] It also includes results from the ATLAS-2M study reporting that cabotegravir/rilpivirine LA injections can be used every two months. [83] About a dozen posters will look at dual ART, largely dolutegravir/lamivudine, including updates from the phase 3 GEMINI and TANGO studies. [84, 85, 86]
Table 1: Recent regulatory approvals and submissions
| Compound / formulation | Class | Approved / submitted |
Company |
| ibalizumab | bNAb. | Approved US: April 2018.
Approved EU: Sep 2018. |
Theratechnologies |
| fostemsavir | gp120 attachment inhibitor. | Submitted US: Dec 2019.
Submitted EU: Jan 2020. |
ViiV Healthcare |
| cabotegravir LA and rilpivirine LA injections | INSTI + NNRTI injections. | Submitted US: Apr 2019
Submitted EU: July 2019 |
ViiV Healthcare Janssen |
| cabotegravir oral | INSTI – oral formulation used for lead-in dosing. | Submitted US: Apr 2019
Submitted EU: July 2019 |
ViiV Healthcare Janssen |
| elsulfavirine, prodrug of VM-1500A | NNRTI – similar activity to efavirenz. Long-acting monthly IM/SC injections. 96-week phase 2 results at AIDS 2018. | Apparently licensed in Russia.
No published phase 3 data or submission to FDA or EMA. |
Viriom |
Table 2: HIV pipeline compounds by development phase (excluding individual bNAbs)
| Compound /Company | Class | Notes | Phase |
| islatravir
(EFdA) Merck/MSD |
NRTTI | Highly potent, low dose, active against NRTI resistance. Long half-life, potential as oral (dosed daily, weekly and perhaps monthly) and an implant (annual). Monthly and annual formulations are for PrEP. | Phase 2/3 |
| islatravir / 3TC / doravirine
Merck/MSD |
FDC: NRTTI + NRTI + NNRTI | FDC with generic 3TC and NNRTI doravirine. Current studies used triple combination for initial ART and switch to islatravir/doravirine for dual maintenance ART. | Phase 3 |
| islatravir/ doravirine
Merck/MSD |
FDC: NRTTI + NNRTI | Dual FDC with NNRTI doravirine. Currently studies look at a switch option after viral suppression with triple drug ART. | Phase 3 |
| GSK3640254
GSK/ViiV Healthcare |
maturation inhibitor | Maturation inhibitor acquired from BMS that has just entered phase 2 studies. Phase 1 results reported in May 2019. | Phase 2 |
| GS-6207
Gilead |
capsid inhibitor | Early stage for new class with activity at multiple stages of viral lifecycle. Sub-cutaneous injection every six months. Phase 1 data presented at IAS 2019 and in press release in November 2019. | Phase 2 |
| elsulfavirine (VM-1500) | NNRTI | Developed by Viriom, already used in Russia as once daily oral drug. Long-acting formulation in develpoment for monthly IM or SC injection. | Phase 2 |
| MK-8504 and MK-8583 | NRTI | Tenofovir prodrugs from Merck, both with completed phase 1 studies. | Phase 1 |
| Combinectin (GSK3732394)
ViiV Healthcare |
entry inhibitor
gp41 and CD4 |
Combined adnectin/fusion inhibitor that stops viral entry by targeting multiple sites of action and the potential for self-administered once-weekly injections. | Phase 1 |
| bNAbs: leronlimab, UB-421, VRC01, 3BNC117, 10-1074,10E8, N6, PGDM1400 and PGT121 etc. | bNAb’s:
Multiple targets include CD4 binding, v3 loop etc |
Many bNAbs are in development for prevention, treatment and cure research – often in long-acting LS formulations and in dual or triple combinations.
Many public and private research institutes (NIH, Rockefeller etc) and pharmaceutical research companies (Gilead, ViiV etc). See Table 3. |
Preclincal, Phase 1 to 3 |
Compounds in development by class
Integrase inhibitors
cabotegravir LA
The 2019 pipeline report summarised the clinical efficacy and safety of long-acting cabotegravir and rilpivirine injections and the regulatory complications are discussed above. [1]
However, the US National Institute for Allergy and Infectious Diseases (NIAID) recently announced a new study using cabotegravir LA (without rilpivirine) in a dual combination with VRC07-523LS – a long-acting bNAb. [16]
This is an open label single arm phase 2 study in 74 participants who are stable on current ART. At baseline all participants will switch to oral cabotegravir and also continue their current NRTIs.
They then switch to cabotegravir LA injections (every four weeks) plus VRC07 infusions (every eight weeks) for 48 weeks. All participants return to regular oral ART for a second year of follow-up. [17]
ViiV also recently announced an agreement to develop another bNAb developed by NIAID called N6LS (see later below). [18]
Cabotegravir is also being developed as a 6-monthly PrEP implant.
NRTIs
islatravir (EFdA)
Islatravir is an NRTI in development by Merck that is notable for vey high potency, a long plasma half-life (~120 hours) that allows weekly and perhaps monthly oral dosing and a slow-release removable implant that might only require annual dosing.
Ongoing studies are for use as both treatment and PrEP. It is classified as a nucleoside reverse transcriptase translocation inhibitor (NRTTI) and has multiple mechanisms of action. [19]
Islatravir has a 4-fold lower IC50 for MK-8591 triphosphate than any other marketed NRTIs with doses of 0.75 mg daily or 10 mg weekly. Common NRTI mutations, including M184I/V, K65R, and K70E, only confer low fold-shifts in potency and islatravir has greater inhibitory quotients against these drug-resistant mutations than those of TDF, TAF, and 3TC with wild-type HIV.
New results on islatravir both as treatment and PrEP were presented at IAS 2019. A phase 2b dose-ranging study randomised 121 treatment naive participants to one of three doses of islatravir (0.25, 0.75 or 2.25 mg daily) plus doravirine and lamivudine or to a control arm of doravirine/3TC/TDF, plus appropriate placebos. [20]
Mean viral load reductions at week-24 in the pooled islatravir arms was –2.9 log copies/mL (with no differences between doses), compared to –2.8 log copies/mL in the control group. Viral load <50 copies/mL at week 24 were reported in 89%, 100%, 87% and 87% in the 0.25, 0.75, 2.25 mg and control arm respectively, but with no protocol-defined viral failures (>200 copies/mL). After 24 weeks, this study then switched participants with undetectable viral load in the islatravir arms to dual therapy with daily islatravir at the 0.75 mg dose plus doravirine. [21]
At week 48, five participants in the islatravir arms (4 rebound, 1 non-response) vs one in the control arm (viral rebound) had viral load levels that were >50 copies/mL. However, no participants had viral load rebound >200 copies/mL and all were reported as being <80 copies/mL.
Phase 3 studies using a two-drug fixed dose combination of doravirine/islatravare already planned or ongoing in people who are treatment-naive, treatment-experienced and drug resistant. [22, 23, 24]
Details on once-weekly oral dosing dual ART, with another long-acting compound in pre-clinical development have not been announced (but see MK-8504 and MK-8683 below).
Islatravir PrEP studies include an ongoing phase 2 study using an oral formulation for once-monthly dosing. [25] If this is effective, it will have the potential to significantly expand the indication for PrEP from a relatively small group of people at high risk of HIV to the billions of people globally who are sexually active but whose HIV risk is much smaller.
IAS 2019 also included results from a randomised phase 1 pharmacology dose finding study of an islatravir implant for PrEP (54 mg and 62 mg) that led to development being taken forward with the higher dose. This provided drug levels above the target threshold of 0.05 pmol/106 cells, with project duration of protection of 12 to 16 months. [26]
Updates from CROI 2020 include a late breaking oral presentation on PrEP efficacy in a macaque study using weekly oral islatravir and two posters related to metabolic outcome and dose selection. [87, 88, 89]
MK-8504 and MK-8583
There are few details on the other long-acting compounds Merck plans to study islatravir with but MK-8504 and MK-8583 are two tenofovir prodrugs studies in HIV positive phase 1 studies.
A single dose of MK-8504 (100 mg or 240 mg) produced reductions in viral load of about –1.0 log (range +0.5 to –1.5) copies/mL. [27]
Results have not yet been published for MK-8583. [28]
Updates from CROI 2020 include a poster on these two compounds reporting on antiviral efficacy from single doses. [90]
GSK NRTTI
GSK have also filed several patents for NRTTI compounds that are currently pre-clinical stages with plans to formulate these as a long acting injection.
bNABs
A growing number of HIV broadly neutralising monoclonal antibodies (bNAbs) are now in development – with more to follow, engineered to improve potency, breadth of coverage and half-life etc. See Table 3.
They generally need to be used in combinations and also require sensitivity testing at baseline to know whether or not they are likely to be active. These sensitivity tests are also in early stage development.
Other first generation bNAbs with early HIV trials (including 2F5, 4E10, 2G12, F105, hNM01, and KD-247) were generally safe in phase 1 studies but have all been superseded by molecules with broader coverae and longer half-lives.
bNAbs target HIV (currently to one of six regions of the virus linked to CD4 attachment) and have the potential to maintain viral suppression after ART has been stopped through both antiviral and immune modulating mechanisms. Long-acting formulations – called LS from the two mutation changes – bring the potential of extended dosing schedules – from 2 to 6 months.
Drug resistance has so far reported for all individual bNAbs, and strategies to reduce this risk include use of combination bNAbs and engineering bispecific or trispecific compounds.
Although there have been few new clinical studies presenting new data since CROI 2019, several new studies have been launched and both ViiV and Gilead have announced licensing rights to develop some of the most promising bNAbs.
The number of studies referenced below for each of the most promising bNAbs show the research interest in this field but is unlikely to be comprehensive. An updated table of these and other compounds is produced by Richard Jefferys at TAG as part of a resource on cure-related trials. [29]
CROI 2020 includes several studies looking at bNAbs in general including baseline susceptibility, tissue penetration (including CSF). Several studies related to aspects of individual compounds in adults and infants, including leronlimab, VRC01, N6-LS and 3BNC117. [91, 92, 93, 94, 95, 96, 97, 98]
Table 3: bNAbs for HIV prevention, treatment or cure research
| Compound / Company |
Target | Notes | Status |
| ibalizumab | gp120 | Already approved in the US and EU for treatment of MDR HIV. | Approved. |
| leronlimab (PRO 140)
CytoDyn |
CCR5 | Once-weekly sub-cutaneous injection being studied in addition to ART for multi-drug resistance and as monotherapy maintenance therapy (without ART). Phase 3. | Phase 3. |
| UB-421
United BioPharma |
CD4 binding | Infusion dosed either weekly or every two weeks as alternative to ART during treatment interruption. Phase 3. | Phase 3. |
| VRC01 and VRC01LS
US NIH |
CD4 binding | Intravenous infusion being studied in cure research and as PrEP (2 large phase 3 studies are ongoing). Sub-cutaneous dosing of infants to prevent transmission at birth or from breastfeeding. VRC01LS is a longer acting formulation. Results as PrEP expected late 2020. | Phase 3. |
| VRC07, VR07-523LS | CD4 binding | Engineered from VRC01. Being studied with cabotegravir-LA in ACTG trial. | Phase 2. |
| PGT-121 and GS-9722 (elipovimab).
Gilead. |
C3/V3 | PGT121 is an IgG1 mAb that targets the V3 Env epitope. GS-9722 (elipovimab) is angineered from PGT-121. | Phase 1. |
| 3BNC117 and 10-1074;
Rockefeller University and Gilead |
CD4 binding and C3/V3 | Both bNAbs are available as LS long-acting formulations.
Gilead Sciences signed for exclusive global development rights. |
Phase 2. |
| N6
US NIH and ViiV |
gp120 | Developed by US NIH and now licenced to ViiV. | Phase 1. |
| Other mAbs: 10E8, trispecific bNAbs, PGDM1400 | MPER, V2 and others | Mulitple compounds in preclinical and phase 1 studies. | Phase 1. |
leronlimab (PRO140)
Leronlimab is a humanised IgG4 monoclonal antibody that blocks HIV entry by binding to CCR5 but is active against maraviroc-resistant virus.
This compound has been in development for more than a decade and has been designated fast-track status as a treatment for MDR HIV. In addition to use as an ARV in combination leronlimab is also being studied as monotherapy after viral suppression on oral ART.
Studies listed as recruiting on the clinical trials registry include a multicentre US phase 2/3 study in 25 treatment-experienced participants. Leronlimab will be given by weekly 700 mg subcutaneous injection, with background ART optimised after the first week and a primary endpoint of >0.5 log reduction in viral load at day 7. [30]
A second much larger US phase 2b/3 study has enrolled more than 550 HIV positive participants on stable ART (viral load <50 copies/mL at screening visit and been on ART for > 6 months). Participants are switched to open-label leronlimab monotherapy given by weekly sub-cutaneous injections at 350 mg, 525 mg or 700 mg doses. However, preliminary results presented at CROI 2019 included a high failure rate (65%; 149/226) in the 350 mg arm (with participants having the option to roll over into a higher dose arm). [31, 32]
According to a more recent press release from August 2019, the rate of viral suppression overall after ten weeks was 68%, 94% and 85% in each of these three arms, respectively. It also states than 150 participants have maintained undetectable viral load out to one year, but it is unclear how many people have reached this endpoint. The study completion date is listed as July 2020. [33]
Leronlimab is also being studied in non-HIV setting as prophylaxis against graft vs host disease (GVHD) in people undergoing allogeneic stem cell transplant. [34]
Leronlimab is being developed by CytoDyn.
CROI 2020 includes a poster on use in four-class resistance. [92]
UB-421
UB-421 is a broadly neutralising mAb that targets CD4 binding. In vitro data suggest comparable or greater potency compared to other compounds, including VRC01 and 3BNC117.
Results from a phase 2 study in 29 virally suppressed participants on ART were presented at CROI in 2017 and published in the New England Journal of Medicine in April 2019. [35]
This used UB-421 monotherapy during an 8-week treatment interruption. UB-421 was given by infusion either 10 mg/kg weekly or 25 mg/kg every two-weeks. Although there were no cases of viral rebound during the monotherapy phase, viral load rebounded at 35 to 62 days after the last UB-421 dose in five participants who delayed restarting ART. All five later restarted ART and viral load became undetectable.
Several studies are listed as expecting to start in 2020. Although these are briefly summarised below, the same studies have been listed since 2017 with the starting dates changing each year.
A phase 2 study in 39 HIV positive participants is listed as being due to start in August 2020, looking at whether adding UB-421 to ART is able to reduce the HIV reservoir compared to ART alone. Entry criteria include already being virally suppressed on current ART. Unlike nearly all other HIV research studies with investigational products. There is effectively no upper age limit (although the range 20 to 100 years old is specified). [36]
A small open label study in 10 treatment-experienced participants with extensive drug resistance is listed as not yet recruiting. [37]
A phase 3 study in 520 HIV positive people with viral suppression on stable ART will randomise participants to either continue ART or stop ART and switch to monotherapy using UB-421 infusions every two weeks. [38]
UB-421 is being developed by United BioPharma with all sites in Taiwan.
No updates are expected at CROI 2020.
VRC01 and VRC01LS
VRC01 is an early broadly neutralising mAb (active against 80-90% HIV strains) that targets the CD4 binding site. It can be given by infusion or sub-cutaneous injection and has been studied in phase 1/2 development with multiple indications: for treatment, prevention and as a component of cure research.
Most ongoing studies are looking at VRC01 for HIV prevention, with two large international dose-finding, placebo-controlled phase 2 studies using VRC01 as PrEP are already ongoing that allow the option for participants to also use open-label oral TDF/FTC PrEP. [39, 40]
Although results are expected in late 2020 there are concerns about using a single mAb given limited breadth and potency from one compound. Modelling suggests that nearly complete neutralisation of a given virus is needed for in vivo protection (~98% neutralisation for 50% relative protection) and that the inclusion of a second bNAb – is likely important to provide cross-clade protection in African studies. [41]
Unfortunately, in a phase 1 study, VRC01 produced no additional impact on reducing the latently infected viral reservoir after being added to ART. VRC01 also had little impact on time to viral rebound after stopping ART, as part of a strategy in cure research.
VRC01 is being studied as part of a dual bNAb combination with 10-1074 in a phase 1 study in 75 HIV positive people on suppressed ART who will be asked to stop HIV treatment. [42]
A phase 1 study is looking at VRC01 with ART in 25 HIV positive people diagnosed during primary infection, with sites in Kenya, Tanzania and Thailand. [43]
A new long-acting formulation – VRC01LS – is also in phase 1 studies, designed to improve the half-life of the antibody, administered IV. This includes using a single injection of VRC01LS in infants after birth to limit risk of vertical transmission and a potential role of additional injections for breastfed infants. [44, 45] Another phase 1 study is looking at responses to VRC07 in eight people who received VRC01LS. [46]
CROI 2020 includes studies on VRC01 tissue penetration and use as infant prophylaxis. [93, 94, 95, 96]
VRC07 and VR07-523LS
VRC07 is an engineered clonal relative to VRC01 and includes VRC07-523LS which is a second-generation bNAb with improved potency, breadth, expression and an LS version to extend the half-life.
Results from a phase 1 safety study in 26 HIV negative participants was published in Lancet HIV in October 2019. [47]
It is now being studied alone and in combination with other bNAbs including 10E8VLS, PGT121 and PGDM140 in at least seven studies, usually phase 1 in HIV negative participants. However, this also includes a study in HIV-exposed infants and at least two studies in HIV positive adults. [45, 48, 49]
A new ACTG study (ACTG 5837) was announced that uses cabotegravir-LA every 4 weeks with VRC07-523LS every two months. [50]
Entry criteria include baseline susceptibility to VRC07-523LS based on IC50 of less than or equal to 0.25 ug/mL and a maximum percent inhibition greater than 98% using the Monogram Phenosense Assay on sample obtained at the screening visit.
NIAID have also granted TaiMed a non-exclusive license to develop VRC07-523LS in combination with the company’s other bNAbs. [51]
No updates are expected at CROI 2020.
PGT-121 and GS-9722 (elipovimab)
PGT121 is an IgG1 mAb that targets the V3 Env epitope. Results of a randomised double blinded, dose escalation, placebo-controlled trial phase 1 study were presented at CROI 2019. [52]
Results in 15 treatment-naive participants, reported that a single infusion of PGT121 produced a median viral load reduction of –1.7 log copies/mL in participants with high baseline viral load, but breakthrough with bNAb resistance also occurred quickly when used as monotherapy. In two people starting with low baseline viral load (<400 copies/mL) a single infusion dropped viral load to undetectable where it remained, without ART, for at least the next six months.
The first part of this study included 20 HIV negative participants. Safety and tolerability was good in both parts of the study with most participants reporting no local or systemic side effects from the single treatment. The only serious adverse event was a knee operation not related to study medication. Grade 2 events (headache and malaise) were reported in approximately 5% participants.
Two phase 1 studies in HIV negative participants are ongoing but a third phase1/2 study with other bNAbs includes HIV positive participants. [49]
PGT-121 was licensed from IAVI/Theraclone to Gilead in 2014 who developed a derivative called elipovimab (GS-9722), which was also reported at CROI 2019. [53] No ongoing or planned studies are currently listed for elipovimab.
Studies at CROI 2020 include new animal data on PGT-121 and looking at the viral reservoir and viral rebound and results from a phase 1 PK study of GS-9722 in HIV positive people. [99, 100, 101, 102]
3BNC117, 10-1074 and LS formulations
3BNC117 targets the CD4 binding site and 10-1074 targets the base of the V3 loop of the HIV envelope protein so in combination there is no cross resistance.
Both bNAbs were developed at Rockefeller University. When used together, a study reported at CROI 2019 reported that 2/13 participants maintained viral load below detection for over a year after interrupting ART, with one person extending this to two years. [54]
Both bNAbs are now engineered into long-acting LS formulations that might enable 6-monthly infusions. It is significant that in January 2020, Gilead announced it had signed licensing agreement for both exclusive and global development rights, although all ongoing studies will continue. No details of the financial settlement were disclosed. [55, 56]
Several phase 1 studies are already ongoing using this combination in HIV positive participants, two of which include treatment interruptions. [57, 58, 59, 60]
A UK placebo-controlled study is also using both long-acting bNAbs to maintain viral suppression during a treatment interruption. The RIO study plans to enroll 75 HIV positive people who were diagnosed during primary infection and who started immediate ART. [61]
A phase 2 study uses 3BNC117 in combination with the fusion inhibitor albuvirtide (approved in China as a one-weekly formulation, similar to enfuvirtide) as maintenance therapy in 80 HIV positive participants on stable ART, in US sites. [62]
Although listed, it is difficult to understand why another small phase 1 study is using 3BNC117 and 10-1074 with pegylated interferon, given the difficulty with side effects.
Studies at CROI 2020 on 3BNC117 include a reservoir study with romidepsin and on 10-1074 include a PK study on infant prophylaxis. [98, 94]
N6 and N6-LS
N6 is a bNAb also developed by the US NIH from the VRC01 class, engineered to have higher potency, active against 98% of isolates tested and also developed into a long-acting LS formulation. [63]
The only study currently listed is an ongoing phase 1 study in 40 HIV negative participants. [64]
In November 2019, ViiV Healthcare announced that it had negotiated an exclusive license to develop N6LS for both treatment and prevention of HIV. [65]
Studies at CROI 2020 include a phase 1 dose escalation study in HIV negative people, a CNS immune activation study in macaques and another looking at dnamics of viral rebound with TLR-7 agonist GS-9620. [97, 99, 100]
Other bNAbs: 10E8, trispecific bNAbs, PGDM1400
Several other promising antibodies are in development.
10E8v2.0/iMab is a bispecific antibody that showed almost 100% neutralisation breadth across a 118-member pseudotyped panel with mean inhibitory concentration of 0.002 ug/mL. It has also prevented infection in mouse studies. [66]
A phase 1 dose escalation study using both IV and subcutaneous formulations includes HIV positive people not yet on ART (as well as HIV negative participants). [67]
However, while the safety and tolerability of bNAbs are generally good, one study using 10E8 was recently put on hold due to grade 3 skin erythema in 7/8 participants. Reactions occurred two days after receiving dual 10E8LS and VRC07 infusion (separately to each side of the stomach). These were associated with mild tenderness and fever (both transient) and confirmed by biopsy as panniculitis with lymphocytic inflammation (all cases resolved). This was sufficient to put further clinical development of 10E8 on hold. [68]
The implications for the triple and trispecific studies that include 10E8 are unclear.
Preliminary results for a trispecific bNAb were presented at CROI 2019. This is the result of a joint development by the Vaccine Research Centre at NIAID and Sanofi where a single molecule could interact with three independent envelope regions: the CD4 binding site, MPER and the V1V2 glycan site. [69]
PGDM1400-1412 is a range of bNAbs with high potency and PGDM 1400 is already included in two recruited ongoing phase 1 studies including HIV positive people in combinations with PGT-121, VRC07-523LS and 10-1074. [70, 71]
A phase 1/2a study is also ongoing in HIV positive people in combination with PGT121, VRC07-523LS and PGDM1400. [49]
Capsid inhibitors
GS-6207
Capsid is the cone-shaped structural core within the HIV virion that protects HIV RNA and related enzymes. The capsid protein (p24) is active in both early and later stages of the HIV lifecycle. It first breaks down to release viral contents into the CD4 cell to enable reverse transcription and also needs to reassemble inside new virions as part of the maturation process at the end of the lifecycle.
GS-6207 binds at a site that blocks both disassembly and assembly, leading to defective new virions that are non-infectious. The compound is potent with EC50 in target cells of 60 to 140 pM (compared to 1000 to 19000 for efavirenz, dolutegravir and atazanavir) with activity against drug resistance to current HIV classes. Although population sequencing showed the binding site to be highly conserved, capsid resistance can be generated from in vitro serial passaging.
Phase 1 results presented at IAS 2019 reported mean –2.2 log reduction at day 10 with GS-6207 monotherapy in treatment-naive participants (at which point ART was started). [72]
Further phase 1 results at EACS 2019 supported development of a modified six-monthly formulation in treatment-experienced participants in phase 2 studies. This will be a sub-cutaneous injection with the potential for self-administration. [73]
The most common side effects were injections site reactions, reported in 19 (59%) participants receiving GS-6207 compared to in 2/8 (25%) of participants receiving placebo. These were all mild and mainly erythema (47%) or pain (38%) and resolved within a few days.
A phase 2 study in 175 treatment naive participants will use oral GS-6207 to cover the first two weeks before using subcutaneous infusion of the capsid inhibitors, with oral F/TAF used throughout. However, when the infusion is repeated after six months, background F/TAF will be switched to either daily oral TAF or daily oral bictegravir. GS-6207 infusions will continue every six months to study these two-drug therapies with the option to continue after week 80. This study is also notable for using bictegravir as a separate single drug formulation. It is already enrolling in US sites with a primary completion date of April 2021. [74]
A second study, also currently enrolling in US sites is a phase 2/3 study to GS-6207 with optimised ART in 100 treatment experienced people with multidrug resistance. The design includes two weeks of functional monotherapy with oral GS-6207 or placebo, before background is optimised for use with 6-monthly subcutaneous GS-6207 plus optimised ART. A second cohort will run for people who don’t meet criteria for initial randomisation, or for when this study is fully enrolled, that will start with optimised ART and subcutaneous GS-6207 from day one. [75]
ViiV Healthcare have a capsid inhibitor in pre-clinical stages with development reference name VH4004280.
Studies at CROI 2020 include an oral presentation and posters on antiviral activity and PK and another poster on resistance, [103, 104, 105, 106]
Maturation inhibitors
GSK3640254
The maturation inhibitor GSK3640254 works at the late stage of the viral lifecycle, by producing non-infectious, undeveloped HIV. As with other new classes, maturation inhibitors will not be cross-resistant to other types of HIV drugs.
Results from two phase 1 studies in HIV negative adults that reported good safety outcomes and bioavailability of two different formulations were presented last year. [76]
These supported further development as a once-daily oral pill.
A proof of concept, phase 2 dose-finding study is ongoing in 34 treatment-naive HIV positive participants. This is a two-part study using monotherapy for 10 days, initially looking at doses of 10 mg and 200 mg, with part two expecting to use, 5 mg, 40 mg and 100 mg, with placebo controls. Dosing is with food. This is an international study with sites in the US, France, Germany, Italy, Spain and South Africa. [77]
GSK3739937 is a second maturation inhibitor compound in preclinical development as a long acting injectable formulation (both subcuteaous and intramuscular).
Both compounds are being developed by GSK/ViiV.
Studies at CROI 2020 include a poster on preclinical development of second generation maturation inhibitors. [107]
Other compounds
Although little data has been reported for the following compounds over the last year (at least), they are still in active development.
Elsulfavirine (VM-1500A)
Elsulfavirine (a prodrug of VM-1500A) is an NNRTI being developed by Viriom that is currently being used in Russia.
Although limited data are available, in a randomised, double-blind phase 2b study conducted in Russia in 120 treatment naive participants, elsulfavirine 20 mg was compared to efavirenz 600 mg, each with TDF/FTC background NRTIs.
The elsulfavirine arm reported similar viral suppression to <50 copies/mL (81% vs 73%), including those with baseline viral load >100,000 copies/mL (78% vs 62%), with fewer CNS side effects (32% vs 62%). [78]
A long-acting injectable formulation is in development, with results from an animal study presented at IAS 2017, showing the potential for monthly by intramuscular (IM) or subcutaneous (SC) injection. [79]
CROI 2020 includes the first safety and PK results for the long-acting formulation of elsulfavirine. [108]
Combinectin (GSK3732394)
Combinectin (GSK3732394) is a biologic combined adnectin/fusion inhibitor that stops viral entry by targeting multiple sites of action on gp41 and CD4. This compound has the potential for self-administered once-monthly subcutaneous injections.
A summary of in vitro activity and resistance data and virologic data from mouse studies were presented at Glasgow 2016. [80]
In June 2019 the first phase 1 study in HIV negative volunteers started enrolling, with results expected mid-2020. [81]
CROI 2020 includes an oral presentation on combinectin. [109]
Postscript: GSK announced in July 2020 that combinectin development was stopped. [113]
ABX464 – Rev inhibitor
ABX464 is an anti-inflammatory molecule thought to work by blocking the end stages of viral assembly.
Although results from a phase 2a dose-ranging study reported 0.5 log copies/mL at day 14 in people using the highest 150 mg dose as monotherapy, HIV research is looking at strategies with compound to reduce the viral reservoir.
The only other going studies related to use for ulcerative colitis, Crohn’s disease and arthritis.
CROI 2020 includes a poster on ABX464 reducing HIV transcription and the viral reservoir. [110]
BIT225 – VPU
BIT225 is currently in phase 2 studies.
It is different to other experimental HIV drugs because it targets cells like macrophages and so is being used in addition to conventional ART to see whether it can reduce the viral reservoir.
CROI 2020 includes a poster on a phase 2 study of BIT225 in addition to ART. [111]
Development stopped or on hold
GS-9131 – NRTI
GS-9131 is an NRTI with good potency (EC50 = 25-200 nM) that has synergistic activity in combination with many other HIV drugs and high in-vitro threshold to resistance that was reported in the previous pipeline report.
However, it is no longer being actively developed by Gilead who are prioritising development of other compounds.
CROI 2020 includes a poster on susceptibility of GS-9131 to drug resistant HIV-2. [112]
GS-PI1 – protease inhibitor
The previous pipeline report included information about GS-PI1, a once-daily unboosted protease inhibitor with high potency and a long half-life.
However, it has since been put on hold to prioritise other compounds in development by Gilead.
Conclusion
Although there are now fewer large companies bringing new HIV drugs to market, Gilead, GSK/ViiV and Merck/MSD all have long-acting molecules that cover both treatment and prevention, see Table 4. They are also all running studies of dual-therapy ART.
Although Janssen are not developing new HIV treatments their HIV vaccine is currently in two large phase 3 studies.
It is notable that these companies are also focused on targeting the HIV reservoir and related cure research.
If successful, the compounds in the pipeline report should dramatically reduce HIV incidence and provide better treatment for people living with HIV who for whatever reason have difficulty with daily oral medicines. (See Table 5).
Table 4: Compounds with long-acting formulations
| Compound | Company |
| cabotegravir | ViiV Healthcare |
| islatravir | Merck/MSD |
| MK-8504 / MK-8583 | Merck/MSD |
| bNAbs: including 3BNC117 and 10-1074; PGDM1400 and PGT121, 10E8. | Various including NIH/NIAID, Rockefeller University, ViiV Healthcare, Gilead Sciences. |
| GS-6207 (capsid) | Gilead Sciences |
| GSK ’937 (maturation) | ViiV Heathcare |
| combinectin | ViiV Heathcare |
| elsulfavirine | Viriom |
Table 5: Likely positioning for new drugs
| Indication | Name |
| treatment-naive | islatravir, doravirine/islatravir |
| switch options on ART | islatravir, doravirine/islatravir, cabotegravir LA/rilpivirine LA, bNAbs, |
| multidrug resistance (MDR) | islatravir, bNAbs, new classes: capsid and maturation inhibitors. |
| PrEP | cabotegravir-LA; islatravir; VRC01, all other mAbs. |
| maintenance without ART | bNAbs – in combinations as switch after viral load suppressed on ART. |
References
References are generally to earlier reports in HIV Treatment Bulletin (HTB). Direct links to the original source documents are included in these reports.
- Collins S. HIV pipeline report. HIV i-Base. July 2019.
https://i-base.info/hiv-pipeline-report-2019. - Collins S. Ibalizumab approved in the EU. HTB (15 November 2019).
https://i-base.info/htb/36786 - Collins S. FDA approves ibalizumab in the US to treat multidrug HIV resistance. HTB (14 March 2018).
https://i-base.info/htb/33659 - Collins S. Fostemsavir submitted to US FDA for multidrug resistant HIV. HTB (13 December 2019).
https://i-base.info/htb/36951 - Collins S. Fostemsavir submitted to EMA for treating multidrug resistant HIV. HTB (29 January 2020).
https://i-base.info/htb/37092 - Collins S. Fostemsavir: 96-week follow-up in people with multi-drug resistance. HTB (24 July 2019).
https://i-base.info/htb/36390 - Collins S. FDA decision on long-acting cabotegravir/rilpivirine (Cabenuva) injections delayed due to scale-up manufacturing problems. HTB (29 January 2020).
https://i-base.info/htb/37064 - Collins S. Phase 3 results with dual therapy cabotegravir/rilpivirine long-acting injections: ATLAS and FLAIR studies. HTB (12 March 2019).
https://i-base.info/htb/35812 - ClinicalTrials.gov. Cabotegravir, CAB) for named patient/compassionate use in HIV.
https://clinicaltrials.gov/ct2/show/NCT03462810 - Study to identify and determine best implementation practices for injectable cabotegravir+rilpivirine in the United States (US).
https://clinicaltrials.gov/ct2/show/NCT04001803 - Khoo S. Long acting parenteral antiretroviral regimens – where might they fit? European HIV Clinical Forum 2019.
https://youtu.be/NyeqYfh6V7Y (webcast)
http://regist2.virology-education.com/presentations/2019/HIVClinicalForum2019/Basel/09_Khoo.pdf (PDF) - ViiV Healthcare submits supplemental new drug application to US FDA for Use of Dovato in Virologically Suppressed Adults with HIV-1
https://viivhealthcare.com/en-gb/media/press-releases/2019/october/viiv-healthcare-submits-supplemental-new-drug-application-to-us-/# - Collins S. Switching to dolutegravir/lamivudine dual therapy is non-inferior to TAF-based triple therapy at week-48 in TANGO study. HTB (24 July 2019).
https://i-base.info/htb/36450 - Dolutegravir/lamivudine approved as dual HIV combination in EU. HTB (17 July 2019).
https://i-base.info/htb/36263 - Collins S. Dolutegravir/lamivudine dual therapy non-inferior to triple ART at week-96. HTB (24 July 2019).
https://i-base.info/htb/36437 - NIAID press release. NIAID launches first clinical trial to test antibody-drug combination for long-acting HIV treatment. (16 January 2020).
https://www.niaid.nih.gov/news-events/antibody-and-drug-combo-trial-long-acting-hiv-treatment - ClinicalTrials.gov. Long-acting cabotegravir plus VRC-HIVMAB075-00-AB (VRC07-523LS) for viral suppression in adults living with HIV-1. NCT03739996.
https://clinicaltrials.gov/ct2/show/NCT03739996 - ViiV press statement, ViiV Healthcare announces exclusive licensing agreement with the National Institutes of Health for investigational “bNAb” with potential for long-acting HIV treatment and prevention. (21 Nov 2019).
https://viivhealthcare.com/en-gb/media/press-releases/2019/november/viiv-healthcare-announces-exclusive-licensing-agreement-with-the - Michailidis E et al. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) Inhibits HIV-1 Reverse Transcriptase with Multiple Mechanisms. J Biol Chem. 2014 Aug 29; 289(35): 24533–24548.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4148878 - Collins S. Islatravir (MK-8591) with doravirine plus lamivudine: 24 week results. HTB (24 July 2019).
https://i-base.info/htb/36398 - Collins S. Dual therapy with islatravir (MK-8591) plus doravirine: 24 week results as switch strategy. HTB (24 July 2019).
https://i-base.info/htb/36443 - ClinicalTrials.gov. Safety and efficacy of a switch to doravirine/islatravir in participants with HIV-1 (MK-8591A-017).
https://clinicaltrials.gov/ct2/show/NCT04223778 - Doravirine/islatravir (DOR/ISL) in heavily treatment-experienced (HTE) participants for HIV-1 Infection (MK-8591A-019).
https://clinicaltrials.gov/ct2/show/NCT04233216 - Randomized, double-blind, efficacy, and safety study of doravirine/islatravir (DOR/ISL) in treatment-naïve participants with HIV-1 infection (MK-8591A-020).
https://clinicaltrials.gov/ct2/show/NCT04233879 - ClinicalTrials.gov. Safety and pharmacokinetics of oral islatravir (MK-8591) once monthly in participants at low risk of HIV-1 infection (MK-8591-016).
https://clinicaltrials.gov/ct2/show/NCT04003103 - 26. Collins S. Islatravir (MK-8591) implant sustains HIV PrEP protection for more than one year. HTB (24 July 2019).
https://i-base.info/htb/36419 - Activity of MK-8504 in antiretroviral-naïve, HIV-1 infected participants (MK-8504-002).
https://clinicaltrials.gov/ct2/show/NCT03188523 - MK-8583 single dose study in HIV-1 infected participants (MK-8583-002).
https://clinicaltrials.gov/ct2/show/NCT03552536 - Jefferys R. Research Toward a Cure Trials. TAG.
https://www.treatmentactiongroup.org/cure/trials - ClinicalTrials.gov. PRO 140 in treatment-experienced HIV-1 subjects.
https://clinicaltrials.gov/ct2/show/NCT03902522 - ClinicalTrials.gov. Study of PRO 140 SC as single agent maintenance therapy in virally suppressed subjects with CCR5-tropic HIV-1 infection.
https://clinicaltrials.gov/ct2/show/NCT02859961 - Dhody K et al. PRO 140 SC: Long-acting, single-agent maintenance therapy for HIV-1 infection. CROI 2019. Poster abstract 486.
http://www.croiconference.org/sessions/pro-140-sc-long-acting-single-agent-maintenance-therapy-hiv-1-infection - CytoDyn press statement. CytoDyn provides update on dose escalating trial with leronlimab for HIV monotherapy for a potential pivotal trial. (14 August 2019).
https://www.cytodyn.com/investors/news-events/press-releases/detail/350/cytodyn-provides-update-on-dose-escalating-trial-with - ClinicalTrials.gov. Study of PRO 140 for prophylaxis of acute GVHD in people with AML or MDS undergoing allogeneic stem-cell transplant. (GVHD). NCT02737306.
https://clinicaltrials.gov./ct2/show/NCT02737306 - Wang C-Y et al. Effect of anti-CD4 antibody UB-421 on HIV-1 rebound after treatment interruption. N Engl J Med 2019; 380:1535-1545. DOI: 10.1056/NEJMoa1802264. (18 April 2019).
https://www.nejm.org/doi/full/10.1056/NEJMoa1802264 - ClinicalTrials.gov. The HIV functional cure potential of UB-421 in ART stabilized HIV-1 participants. NCT03743376.
https://clinicaltrials.gov/ct2/show/NCT03743376 - ClinicalTrials.gov. UB-421 combine with optimized background therapy regimen in multi-drug resistant HIV-1 infection participants. NCT03164447.
https://clinicaltrials.gov/ct2/show/NCT03164447 - ClinicalTrials.gov. To investigate the efficacy and safety of UB-421 monotherapy in HIV-1 infected adults. NCT0314921.
https://clinicaltrials.gov/ct2/show/NCT0314921 - ClinicalTrials.gov. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection in women. NCT02568215.
https://www.clinicaltrials.gov/ct2/show/NCT02568215 - ClinicalTrials.gov. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection among men and transgender persons who have sex with men. NCT02716675.
https://www.clinicaltrials.gov/ct2/show/NCT02716675 - Wagh K et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathogens. March 5, 2018. DOI: 10.1371/journal.ppat.1006860.
http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1006860 - ClinicalTrials.gov. Combination therapy with VRC-HIVMAB060-00-AB (VRC01) and 10-1074 in HIV-infected individuals undergoing sequential treatment interruptions. NCT03831945.
https://clinicaltrials.gov/ct2/show/NCT03831945. - ClinicalTrials.gov. Safety and virologic effect of a human monoclonal antibody (VRC01) administered intravenously to adults during early acute HIV infection.
https://clinicaltrials.gov/ct2/show/NCT02591420 - McFarland E et al. Safety and pharmacokinetics of monoclonal antibody, VRC01LS, in HIV-exposed newborns. CROI 2019. Oral abstract 45.
http://www.croiconference.org/sessions/safety-and-pharmacokinetics-monoclonal-antibody-vrc01ls-hiv-exposed-newborns - ClinicalTrials.gov. Evaluating the safety and pharmacokinetics of VRC01 and VRC01LS, potent anti-HIV neutralizing monoclonal antibodies, in HIV-1-exposed infants. NCT02256631.
https://clinicaltrials.gov/ct2/show/NCT02256631 - ClinicalTrials.gov. Safety and virologic effect of a human monoclonal antibody, VRC-HIVMAB080-00-AB (VRC01LS), with broad HIV-1 neutralizing activity, administered intravenously to HIV-positive adults. NCT02840474.
https://clinicaltrials.gov/ct2/show/NCT02840474. - Gaudinski MR et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV, 6(10);PE667-E679. (01 October 2019).
https://www.thelancet.com/journals/lanhiv/article/PIIS2352-3018(19)30181-X/fulltext - ClinicalTrials.gov. A phase 1, single dose study of the safety and virologic effect of an HIV-1 specific broadly neutralizing human monoclonal antibody, VRC-HIVMAB080-00-AB (VRC01LS) or VRC-HIVMAB075-00-AB (VRC07-523LS), administered intravenously to HIV-infected adults.
https://clinicaltrials.gov/ct2/show/NCT02840474 - ClinicalTrials.gov. A phase 1/2a study of PGT121, VRC07-523LS and PGDM1400 monoclonal antibodies in HIV-uninfected and HIV-infected adults.
https://clinicaltrials.gov/ct2/show/NCT03721510 - ClinicalTrials.gov. Long-acting cabotegravir plus VRC-HIVMAB075-00-AB (VRC07-523LS) for viral suppression in adults living with HIV-1. NCT03739996.
https://clinicaltrials.gov/ct2/show/NCT03739996 - TaiMed press statement. License agreement with NIAID for VRC07-523LS broadly neutralizing antibody. (2 October 2019).
http://www.taimedbiologics.com/news/info/85 - Collins S. First phase 1 results from bNAb PGT121 in HIV positive people. HTB 28 March 2019).
https://i-base.info/htb/35947 - Thomsen ND et al. 356 GS-9722: First-in-class effector-enhanced broadly neutralizing antibody for HIV cure. CROI. 4–7 March 2019, Seattle, Washington. Poster abstract 356.
http://www.croiconference.org/sessions/gs-9722-first-class-effector-enhanced-broadly-neutralizing-antibody-hiv-cure - Collins S. bNAb research at CROI 2019: vaccine, prevention, treatment and cure… HTB (30 April 2019).
https://i-base.info/htb/36040 - Collins S. Gilead announces licensing agreement for Rockefeller University bNAbs. HTB (29 January 2020).
https://i-base.info/htb/37084 - Gilead press statement. Gilead Sciences licenses portfolio of HIV antibodies from the Rockefeller University.
https://www.gilead.com/news-and-press/press-room/press-releases/2020/1/gilead-sciences-licenses-portfolio-of-hiv-antibodies-from-the-rockefeller-university - Combination therapy with 3BNC117 and 10-1074 in HIV-infected individuals.
https://clinicaltrials.gov/ct2/show/NCT03571204 - 3BNC117-LS and 10-1074-LS in viremic HIV-infected individuals.
https://clinicaltrials.gov/ct2/show/NCT04250636 - 3BNC117 and 10-1074 in ART-treated Individuals.
https://clinicaltrials.gov/ct2/show/NCT03526848 - First-in-human study of 10-1074-LS alone and in combination with 3BNC117-LS.
https://clinicaltrials.gov/ct2/show/NCT03554408 - The RIO study. Personal communication with Professor Sarah Fidler, principal investigator.
- Albuvirtide and 3BNC117 as long-acting maintenance therapy in virologically suppressed subjects.
https://clinicaltrials.gov/ct2/show/NCT03719664 - Huang J et al. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity (2016) 45(5):1108–21. doi:10.1016/j. immuni.2016.10.027.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5770152 - VRC 609: A phase 1, open-label, dose-escalation study of the safety and pharmacokinetics of a human monoclonal antibody, VRC-HIVMAB091-00-AB (N6LS), administered intravenously or subcutaneously with or without recombinant human hyaluronidase PH20 (rHuPH20) to healthy adults.
https://clinicaltrials.gov/ct2/show/NCT03538626 - ViiV press statement. ViiV Healthcare announces exclusive licensing agreement with the National Institutes of Health for investigational “bNAb” with potential for long-acting HIV treatment and prevention. (21 November 2019).
https://viivhealthcare.com/en-gb/media/press-releases/2019/november/viiv-healthcare-announces-exclusive-licensing-agreement-with-the - Huang Y et al. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell (2016) 165(7):1621–31. doi:10.1016/j.cell.2016.05.024
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4972332 - 10E8.4/iMab Bispecific Antibody in HIV-uninfected and HIV-infected Adults
https://clinicaltrials.gov/ct2/show/NCT03875209 - Koup R. Review of bNAbs in clinical development. R4P2018, 21-25 October 2018. HVTN and HPTN satellite session. 21 October, 4.00 pm.
http://webcasts.hivr4p.org/console/player/40515 (webcast) - Pegu A et al. Potent antiviral activity of trispecific broadly neutralizing HIV antibodies. Conference on Retroviruses and Opportunistic Infections (CROI), 4-7 March 2019, Seattle. Late breaker oral abstract 28 LB.
http://www.croiconference.org/sessions/potent-antiviral-activity-trispecific-broadly-neutralizing-hiv-antibodies (abstract)
http://www.croiwebcasts.org/p/2019croi/28 (webcast) - Evaluating the safety, tolerability, pharmacokinetics, and antiviral activity of combinations of monoclonal antibodies PGT121, PGDM1400, 10-1074, and VRC07-523LS administered via intravenous infusion in healthy, hiv-uninfected adult participants.
https://clinicaltrials.gov/ct2/show/NCT03928821 - A clinical trial of PGDM1400 and PGT121 and VRC07-523LS monoclonal antibodies in HIV-infected and HIV-uninfected adults.
https://clinicaltrials.gov/ct2/show/NCT03205917 - Collins S. First viral load results for capsid inhibitor GS-6207: mean –2.2 log reduction at day 10. HTB 24 July 2019.
https://i-base.info/htb/36383 - Collins S. Capsid inhibitor GS-6207: potential for 6 monthly dosing for MDR HIV. HTB 13 December 2019.
https://i-base.info/htb/36978 - ClinicalTrials.gov. Study to evaluate the safety and efficacy of GS-6207 in combination with other antiretroviral agents in people living with HIV.
https://clinicaltrials.gov/ct2/show/NCT04143594 - ClinicalTrials.gov. Study to evaluate the safety and efficacy of GS-6207 in combination with an optimized background regimen in heavily treatment experienced participants living with HIV-1 infection with multidrug resistance.
https://clinicaltrials.gov/ct2/show/NCT04150068 - Joshi S et al. The initial phase 1 evaluation of the safety, tolerability, and pharmacokinetics of GSK3640254, a next generation HIV maturation inhibitor, as assessed in healthy subjects. 20th International Workshop on Clinical Pharmacology of HIV, Hepatitis & Other Antiviral Drugs. May 14-16, 2019. Noordwijk, the Netherlands. Abstract 4.
http://regist2.virology-education.com/abstractbook/2019/abstractbook_20ANTIVIRAL.pdf (PDF)
http://regist2.virology-education.com/presentations/2019/20AntiviralPK/10_Joshi.pdf (Slides) - ClinicalTrials.gov. A proof of concept study of GSK3640254 in HIV-1 infected treatment-naive adults. NCT03784079.
https://clinicaltrials.gov/ct2/show/NCT03784079 - Bichko V et al. Pre-clinical pharmacokinetics of elsulfavirine/VM1500A long acting injectable formulations. IAS 2017, 23-26 July, Paris. Poster abstract WEPEA0190. http://programme.ias2017.org/Abstract/Abstract/1515
- Scherrer D et al. Early evidence of antiviral activity & safety of ABX464 in HIV treatment-naïve participants. CROI 2016, February 22–25, Boston, Late breaker poster abstract 461LB.
http://www.croiconference.org/sites/default/ les/posters-2016/461LB.pdf (PDF) - Krystal M et al. HIV combinectin GSK3732394: a long-acting inhibitor with multiple modes of action. Glasgow 2016.
http://www.natap.org/2016/GLASGOW/GLASGOW_27.htm - ClinicalTrials.gov. Evaluation of the safety, tolerability and pharmacokinetics (PK) of GSK3732394 first-time-in-human (FTIH) study.
https://clinicaltrials.gov/ct2/show/NCT03984812CROI 2020 references 82 to 111. All references below refer to the Programme and Abstracts of the Conference on Retroviruses and Opportunistic Infections, 8 – 11 March 2020.
http://www.croiconference.org/abstracts/search-abstracts
- DeJesus E et al. Comparable efficacy of ibalizumab in combination with 1 or 2 fully active agents. CROI 2020, Boston. Poster abstract 507.
- Overton ET et al. Cabotegravir + rilpivirine every 2 months is noninferior to monthly: ATLAS-2M study. CROI 2020, Boston. Oral abstract 34.
- Underwood M et al. DTG+3TC vs DTG+TDF/FTC (GEMINI 1&2): confirmed virologic withdrawals through week 96. CROI 2020, Boston. Poster abstract 483.
- Wang R et al. Assessing the virologic impact of archived resistance in an HIV-1 switch study, TANGO. CROI 2020, Boston. Poster abstract 489.
- de Miguel R et al. Long-term DTG-3TC switch efficacy in participants with archived 3TC resistance. CROI 2020, Boston. Poster abstract 485.
- Markowitz M et al. Weekly oral islatravir provides effective PEP against IV challenge with SIVMAC251. Late breaker oral abstract 89LB.
- McComsey GA et al. Islatravir metabolic outcomes in phase IIB trial of treatment-naive adults with HIV-1. CROI 2020, Boston. Poster abstract 686.
- Rudd DJ et al. Modeling-supported islatravir dose selection for phase III. CROI 2020, Boston. Poster abstract 462.
- Matthews RP et al. MK-8504 and MK-8583 (tenofovir prodrugs) single-dose PK and antiviral activity in HIV infection. CROI 2020, Boston. Poster abstract 468.
- Stefic K et al. Susceptibility to bNAbs of transmitted HIV variants among recent infections in France. CROI 2020, Boston. Poster abstract 525.
- Rusconi S et al. PRO 140 in vitro activity in HTE subjects with a 4-class drug-resistant HIV-1 virus. Poster abstract 524.
- Prabhakaran M et al. Infiltration of bNAb VRC01 into the cerebrospinal fluid in humans in the RV397 study. CROI 2020, Boston. Poster abstract 453.
- Capparelli EV et al. Safety and pharmacokinetics of intravenous VRC01LS and 10-1074 in young children. CROI 2020, Boston. Late breaker 465LB.
- Dugdale C et al. Cost-effectiveness of broadly neutralizing antibodies for infant HIV prophylaxis. CROI 2020, Boston. Poster abstract 777.
- Henrich TJ et al. Whole-body PET imaging of the HIV reservoir using radiolabeled VRC01. CROI 2020, Boston. Oral abstract 72.
- Widge AT et al. A phase I dose-escalation trial of human monoclonal antibody N6LS in healthy adults. CROI 2020, Boston. Poster abstract 508.
- Gruell H et al. A randomized trial of the impact of 3BNC117 and romidepsin on the HIV-1 reservoir. CROI 2020, Boston. Oral abstract 38.
- Hsu DC et al. Impact of GS-986, PGT121 and N6-LS on CNS immune activation in SHIV-infected macaques. CROI 2020, Boston. Poster abs343.
- Hsu DC et al. Delay in viral rebound with TLR7 agonist, N6-LS, and PGT121 in SHIV-infected macaques. CROI 2020, Boston. Oral abs 77.
- Barouch D et al. PGT121 and vesatolimod in chronically treated SHIV-infected rhesus monkeys. CROI 2020, Boston. Late breaker poster 345LB.
- Ruane P et al. Safety & pharmacokinetics of GS-9722 in HIV-negative
participants and people with HIV. CROI 2020, Boston. Oral abstract 39. - McDonald C et al. Dose-response relationship of subcutaneous long-acting HIV capsid inhibitor GS-6207. CROI 2020, Boston. Oral abs 69.
- Daar E et al. Dose-response relationship of subcutaneous long- acting HIV capsid inhibitor GS-6207. CROI 2020, Boston. Poster abstract 469.
- Begley R et al. PK, food effect, and safety of oral GS-6207, a novel HIV-1 capsid inhibitor. CROI 2020, Boston. Poster abstract 470.
- Margot NA et al. Absence of GS-6207 phenotypic resistance in HIV Gag cleavage site and other mutants. CROI 2020, Boston. Poster abs 529.
- Ablan S et al. Preclinical development of second generation HIV-1 maturation inhibitors. CROI 2020, Boston. Poster abstract 505
- Yakubova E et al. Safety and PK study of VM-1500A-LAI, a novel long-acting injectable therapy for HIV. CROI 2020, Boston. Late breaking poster abstract 473LB
- Wensel D et al. Structural analyses of a bound anti-cd4 adnectin inhibitor of HIV-1. CROI 2020, Boston. Oral abstract 20.
- Moron-Lopez S et al. ABX464 decreases the total HIV reservoir and HIV transcription initiation in vivo. CROI 2020, Boston. Poster abstract 335.
- Avihingsanon A et al. Phase 2 trial of VPU inhibitor BIT225 in combination with antiretroviral therapy. CROI 2020, Boston. Poster abstract 506.
- Q et al. Susceptibility of NRTI-resistant HIV-2 isolates to a new NRTI, GS-9131. CROI 2020, Boston. Poster abstract 530.
- GSK press release. Second quarter 2020 report. (29 July 2020).
https://www.gsk.com/media/6064/q2-2020-results-announcement.pdf (PDF)

