Importance of rapid PEP: predicting impact of delay and duration of 2- and 3-drug PEP
23 November 2025. Related: Journal scan, HIV prevention and transmission.
Simon Collins, HIV i-Base
A modelling analysis predicting the impact of time taken to initiate PEP was published in a supplement to JIAS. [1]
The results include using either 2-drug or 3-drug PEP and are for continuing PEP for 2 weeks vs 4 weeks.
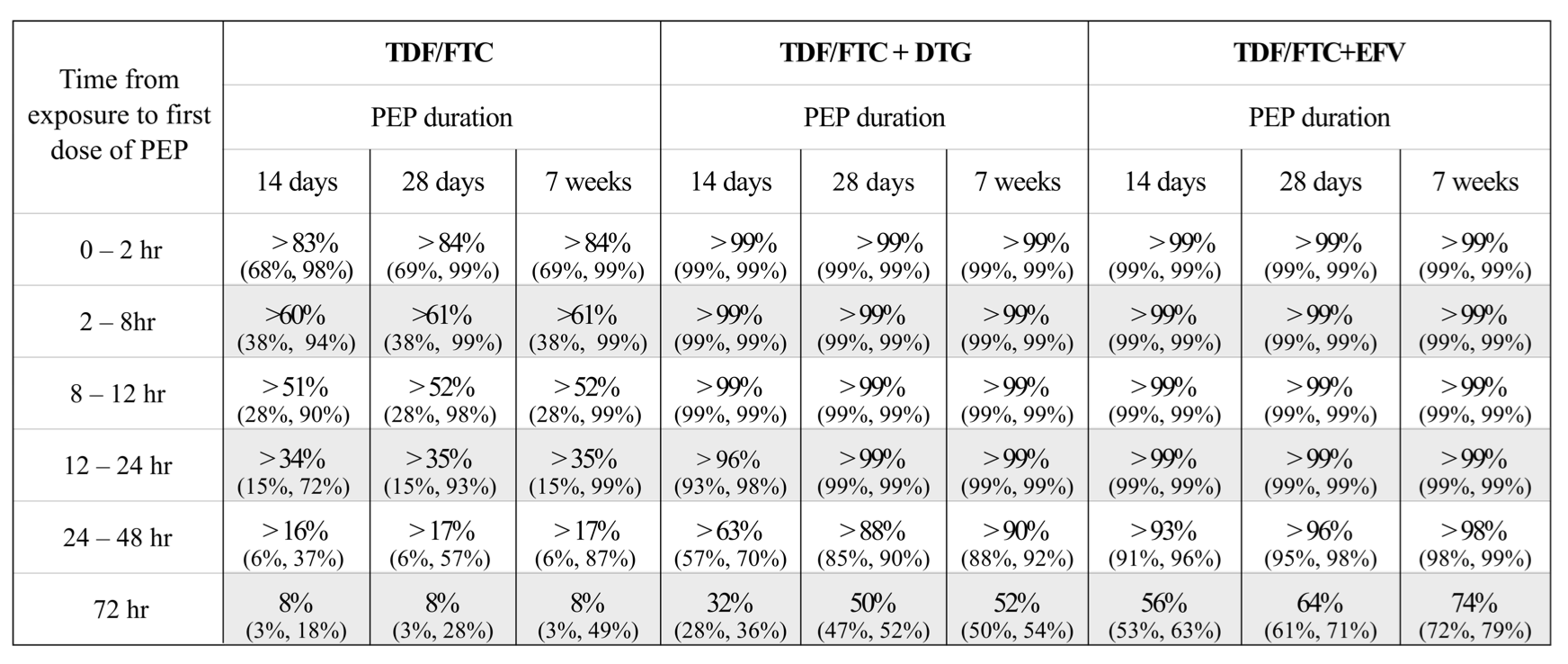
The paper includes a useful practical table (see below) that estimates subsequent PEP efficacy, including 95% confidence intervals. It also shows no impact from continuing PEP out to 7 weeks.
For example, for 2-drug PEP to be effective it really needs to be started within an hour (assuming PrEP was not recently used).
By contrast, 3-drug PEP is still highly effective even if the first dose is delayed by 24 hours, and even if PEP is only continued for 2 weeks. Four weeks of 3-drug PEP remains 88% effective if started between 24-48 hours, but drops to 50% efficacy if not started until 72 hours after sex.
The study also supported the impact of starting PEP with an initial dose of PrEP, as already introduced in the recent UK PrEP guidelines. [2]
Although only modelling data, these results support further clinical research into easier options for shorter course PEP. The study also shows limited differences between dolutegravir- vs efavirenz-based PEP, even though efavirenz is not a recommended option for PEP.
It would be interesting to see the 2-drug PEP modelling use an initial double-dose because of the significant pharmacokinetic advantages. [2]
Table 1. Impact on delays of initiating PEP with 2- and 3-drug combinations
Note: Simon Collins is also a co-author of the JIAS paper.
References
- Zhang L et al. Modelling the impact of initiation delay, duration and prior PrEP on the efficacy of post-exposure prophylaxis containing a tenofovir/emtricitabine backbone. J Int AIDS Soc. Volume 28 Issue Supplement 1: PEP in Africa: prospects, opportunities and challenges. 28: e26454. 26 June 2025. DOI: 10.1002/jia2.26454
https://onlinelibrary.wiley.com/doi/10.1002/jia2.26454 - BASHH/BHIVA. Guideline on the Use of HIV Pre-exposure Prophylaxis (2025)
https://www.bashh.org/resources/5/hiv_preexposure_prophylaxis_2018 [sic]
https://www.bashh.org/_userfiles/pages/files/prep_2025.pdf (PDF)