Question
How good is the monkeypox vaccine?
5 August 2022. Related: All topics, Complications and coinfections, Opportunistic infections, Sexual health.
Hi there, I am an HIV positive man living in London and my clinic offered me the monkeypox vaccine. How good is it and will it protect me?
I am otherwise doing well on ART, with a good CD4 count. I like to enjoy life, including the chance to meet people in different settings. Right now though I am still being cautious.
Answer
IMPORTANT NOTE: This page will be updated as more data becomes available. And also if experts agree on information that should be given with the vaccine,
 Thanks for your questions which lots of other people are asking about.
Thanks for your questions which lots of other people are asking about.
The monkeypox (MPX) vaccine is called Imvanex (in the UK) and called Imvamune, Jynneos and MVA in other countries.
This is especially important as some London clinics run weekend clinics to provide the vaccine.
The quick answer is that the MPX vaccine is highly effective and is very safe. But this is based on results two weeks after after receiving the second dose.
If your clinic contacted you, they recognise that you could especially benefit from the vaccine.
The detailed Q&A below is just as important though. It explains how long the vaccine might take to work, and the likely protection after just one shot.
It also explains why a second shot might be more important if you are HIV positive. However, different studies do not always agree. This uses a cautious approach based on studies in people living with HIV.
The main point is to show that protection takes time to develop and that HIV status might affect this.
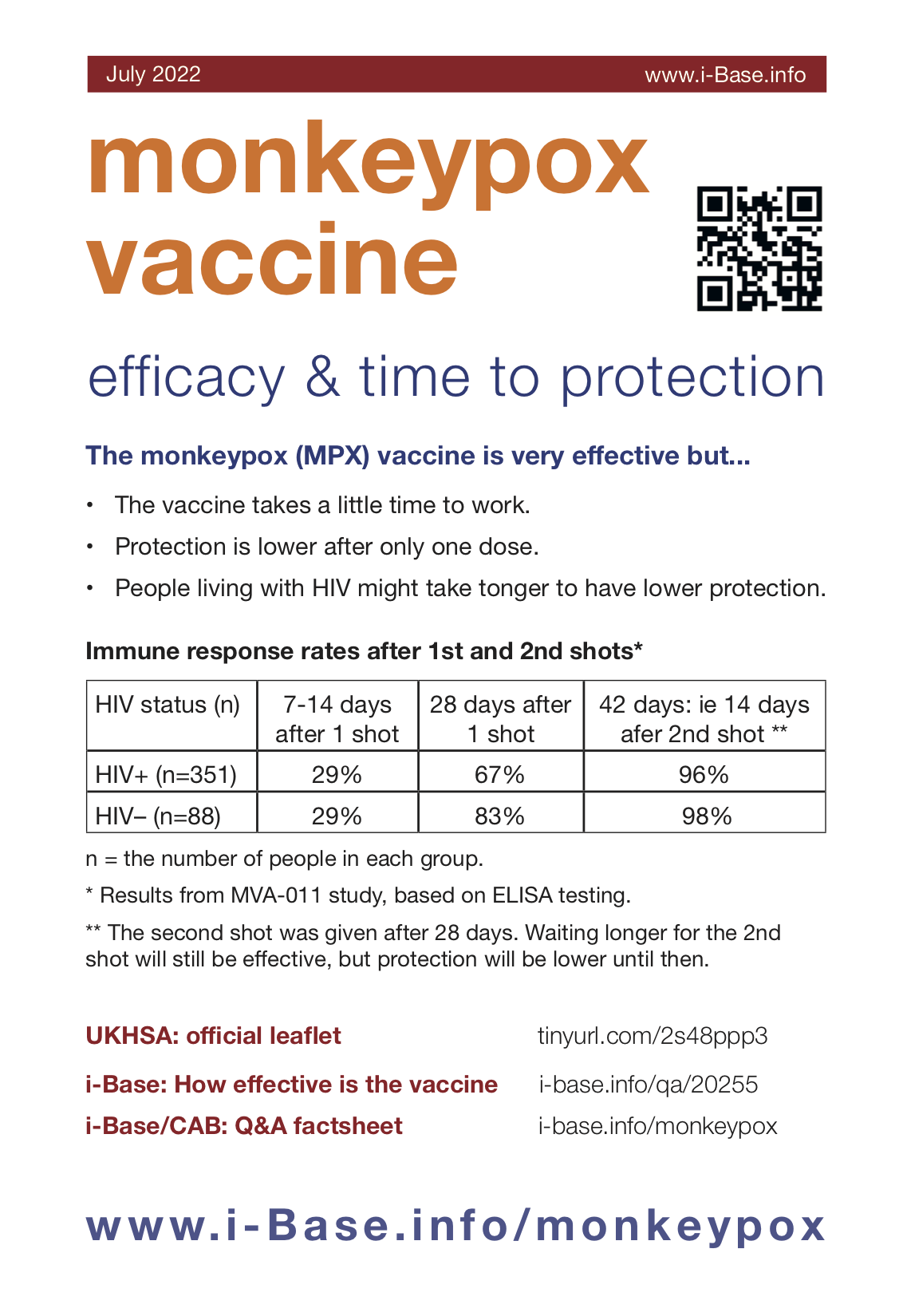
Table 1: Immune response rates after 1st and 2nd shots*
| HIV status (n) | 7-14 days after 1 shot | 28 days after 1 shot | 42 days: ie 14 days after 2nd shot ** |
| HIV+ (n=351) | 29–80%% | 67–80% | 96% |
| HIV– (n=88) | 29–80% | 83% | 98% |
* Results from MVA-011 study, based on ELISA testing. The range of responses is related to how the results have been reported by different sources.
** The second shot was given after 28 days. Waiting longer for the 2nd shot will still be effective, but protection will be lower until then.
n = the number of people in each group.
How effective is the MPX vaccine?
 The MPX vaccine is thought to be highly effective after two shots, 28 days apart.
The MPX vaccine is thought to be highly effective after two shots, 28 days apart.
For example, based on study above, the vaccine is likely to be more than 95% effective two weeks after the second shot. (See Table 1).
But this is based on two shots and then waiting two weeks for the full immune response to develop.
However, the vaccine might be more effective, earlier. A similar size phase 3 study reported approximately 6% protection after one week and 91% after two weeks. This study did not include people living with HIV. [5]
The published HIV studies are included in the references. [6, 7]
Until experts agree, i-Base would prefer to be cautious rather than hear about people developing MPX before protection had developed.
How effective is the MPX vaccine after one shot?
Because there is a limited supply of vaccines the current UK programme is based on only giving one shot. The government say this is a fairer way to meet the high demand.
However, this is an important question because vaccine responses are likely to be lower after only one shot. This also depends on waiting for the vaccine to work.
After 4 weeks, protection after a single shot increases to between 67% to 88%.
These results are based on immune tests called ELISA, but different rates were reported based on other tests. (See Table 1).
How quickly do immune responses develop?
It is likely to take at least 2 weeks for the vaccine to work in most people.
What about if I am HIV positive?
It might take a little longer for the vaccine to work in people living with HIV. We might also need a second dose to get the same protection as someone who is HIV negative.
Data on vaccine protection comes from six main studies.
One of these studies included 351 people living with HIV and 88 people who were HIV negative. CD4 counts were all >200 cells/mm3 in the HIV positive group. (See Table 1).
After 7 to 14 days, immune responses were only reported in about one third (29%) for both groups. This is why waiting for protection after the vaccine is important.
After 28 days, immune responses increased to 67% in the HIV positive group and 83% in the HIV negative group.
After 42 days, two weeks after the second shot, protection increases to 96% in the HIV positive and 98% in the HIV negative groups.
This supports the importance of having a second shot, especially if you are HIV positive.
Is is worth having a single shot?
If you are offered the vaccine or register online to have a first shot, this is strongly recommended.
A single shot will give some protection.
More importantly, you will get full protection more quickly, once the second shot becomes available.
Is the above information being given to people having the vaccine?
For some reason, the information currently being given to people in the UK does not include the details above. [1]
Even though this evidence is limited, this could still be explained and it would help.
It is however in the full prescribing information. [2]
So the information in the Q&A is based on antibody responses to the Imvanex vaccine by ELISA. Although vaccines can work in other ways, this is the main way the vaccines against COVID were reported.
The US CDC also cautions for people to wait six week – ie until two weeks after the second dose. It provides no infoemation about only receiving one dose. [3]
How easy is MPX to transmit or receive?
MPX can be very easy to catch. This includes in situations where it is easy to have sex with anonymous or multiple partners. For example, in private parties, saunas, darkrooms and cruising areas.
Even if you don’t have sex, intimate close contact can easily transmit MPX. This includes from someone touching you (if MPX is on their hands), from kissing and from oral sex.
Rates of MPX are especially high in London, but have now been reported across the UK.
More information
Monkeypox Q&A
https://i-base.info/monkeypox/
More details on transmission, symptoms, treatment and other information.
References
- UKHSA. Protecting you from monkeypox – information on the smallpox vaccination. (8 July 2022). [Accessed 22 July 2022, updated 1 August 2022]
https://www.gov.uk/government/publications/monkeypox-vaccination-resources/protecting-you-from-monkeypox-information-on-the-smallpox-vaccination - EMA. Full prescribing information for Imvanex vaccine. (22 July 2022).
https://www.ema.europa.eu/en/medicines/human/EPAR/imvanex (webpage link)
https://www.ema.europa.eu/en/documents/product-information/imvanex-epar-product-information_en.pdf (direct PDF link) - US CDC. Considerations for monkeypox vaccination. (30 June 2022).
https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html - US Office of Vaccines Research and Review (OVRR). Review memorandum for Jynneos vaccine. (2018)
https://www.fda.gov/media/131870/download - Pittman PR et al. Phase 3 efficacy trial of modified vaccinia ankara as a vaccine against smallpox. N Engl J Med 2019; 381:1897-1908. DOI: 10.1056/NEJMoa1817307
https://www.nejm.org/doi/full/10.1056/NEJMoa1817307 - Overton ET et al. Safety and immunogenicity of modified vaccinia ankara-Bavarian Nordic smallpox vaccine in vaccinia-naive and experienced HIV-positive individuals: an open-label, controlled clinical phase 2 trial. Open Forum Infect Dis. 2015 May 5;2(2):ofv040. doi: 10.1093/ofid/ofv040. Erratum in: Open Forum Infect Dis. 2016 Jan;3(1):ofv183.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4567089 - Greenberg RN et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis. 2013 Mar 1;207(5):749-58. doi: 10.1093/infdis/jis753. Epub 2012 Dec 7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3611764/ - BHIVA rapid guidance on monkeypox virus. (Updated 17 August 2022).
https://www.bhiva.org/BHIVA-rapid-guidance-on-monkeypox-virus
This Q&A was first posted on 23 July 2022. It was last updated on 18 August 2022 to include the updated BHIVA statement. The table now includes a wide range of 2- and 4-week responses.


Hi Matt – thanks so much – great answer.
Finding a way through the studies is difficult. You are right that the table shows the percentage of people who developed antibody responses at different time points after the vaccine.
Some studies shwo higher or lower levels, so this is really to show the benefit of waiting a little while.
We are in touch with the UKHSA about the information in their leaflet. As a result they have updated their info this week to say that the vaccine “should reach the highest protection by about 4 weeks.”
https://www.gov.uk/government/publications/monkeypox-vaccination-resources/protecting-you-from-monkeypox-information-on-the-smallpox-vaccination
Tino the content here does not refer to the efficacy of a single dose in preventing symptomatic infection.
There have not been human trials challenging vaccinated people with live monkeypox virus, so other measures are being used to estimate protection levels over time.
The table here looks at the amount of time after vaccination to find a certain threshold of neutralizing antibodies has built up in the blood.
It is a cautious estimate, since a lower level of antibodies below this threshold may still be effective, the body could quickly respond to exposure by producing more antibodies before symptoms appear, and other forms of immune response other than antibodies could be able to fight the virus earlier on.
We do know that vaccination even post-exposure is able to reduce or prevent symptomatic infection, and we know that animal trials have found the vaccine to prevent infection even when they are immediately challenged with very high levels of the virus.
In other words, this is the most cautious finding to be weighed against other more optimistic findings.
Hi Mark, yes you are right. Other studies, often much smaller ones show a wide range of values.
I wanted to include the MVA-011 study though because is has so many HIV+ participants. This makes it a very important study for us – as well as being one that the EMA selected as important.
The thing I take from the results is the direction of protection increases in all studies over time. So even if we are not exactly sure of the percentage at weeks 1 or 2, it gets stronger in all studies by week 4.
The direction also shoes even higher protection at two weeks after the second dose. So even though the limited vaccine supplies are going to delay the second dose for most people, this is when protection get about 95%.
The more discussion on this issue the better. It is really important for giving people info about how long to wait after the vaccine before returning to regular life.
The official UK information for the vaccine now says that the vaccine “should reach the highest protection by about 4 weeks.”
https://www.gov.uk/government/publications/monkeypox-vaccination-resources/protecting-you-from-monkeypox-information-on-the-smallpox-vaccination
Hello, I’m looking at the chart from which this data was pulled and it looks like there’s a wide range of seroconversion rates at the 7/14 day mark for hiv- or other control individuals (from 70.9%-12.5%), seemingly based on the clinical trial. Are we able to explain why there’s such a range? Or maybe I’m misinterpreting the data? Sorry this may be out of focus for this site but this post is getting a lot of traction outside of this community. Thanks!
Hi Lee. thanks. You are right that on a population level an 80% vaccine should help reduce MPX numbers. But this is also affected by how people change their behaviour based on their risk.
If most people are already avoiding high risk situations, their risk is currently very low. If after the vaccine they go back to situations with high MPX risk, then overall, MPX cases could start increasing again. This is because 20% of people will be back at risk again.
It will also depend on how effective the vaccine is at stopping transmission, rather than just reducing symptoms. The increase in immune respponses is really encouraging, but we don’t know what level of antibodies we need to stop infection.
80+% after 28 days isn’t effective? If everyone was vaccinated with one dose now, you’d cut transmission and the number infected significantly a month from now, even if it’s not fully effective, you’ll at least slow it down and break transmission chains
Hi Peter – thanks for your comment.
The Q&A focussed on the MVA-011 study because roughly 30-50% of the outbreaks in different setting are in people living with HIV. This website is about HIV treatment and care and HIV is often linked to a lower response from vaccines in general. This
This study was selected by both the EMA and FDA when they approved the vaccine.
The vaccines are very good – even after one shot, but immune protection looks higher after 4 weeks than after 2.
Any other help with interpreting these results is appreciated.
Why is the data above just from MVA-011 and did not include other studies? I realize that study did compare HIV – and +, but there was lots of data for HIV – folks. Note that this study had data from 7 days, not 14 days post vax. It’s also 30% for HIV –
Hi Tino, thanks, you make good points.
Firstly, the main aim for the single dose might mainly be to reduce severe symptoms rather than prevent transmission. It is still good to get the vaccine but we don’t know how good a single dose is yet at preventing infections.
Secondly, vaccines alone is unlikely to have much impact on the UK epidemic – especially because there are two few vaccines right now. It will also involve people reducing the the highest risks for catching MPX.
Hi,
Given the poor efficacy of a single dose in preventing symptomatic infection, it is unlikely the current UK strategy will break the chains of transmission.
A single dose could reduce the severity of the illness if infected, which might have a beneficial effect on the 10% of infections that require hospitalisation, but I don’t see how else a single dose strategy can radically alter the course of the epidemic.