PK studies reveal significant sex/gender differences
1 May 2003. Related: Conference reports, Women's health, PK and drug interactions, PK Workshop 4th Cannes 2003.
Steve Taylor and Polly Clayden, HIV i-Base
Gender differences in nevirapine and efavirenz PK. Fact or fiction?
A presentation from Charles la Porte from the University of Nijmegen investigated possible differences between the sexes in the PK of the non-nucleoside reverse transcriptase inhibitors (NNRTIs) nevirapine (NVP) and efavirenz (EFV) [1]. This Dutch group have previously reported gender-related differences in the PK of the PIs indinavir and lopinavir [2,3] and have additionally observed high plasma levels of NVP and EFV in female patients through their TDM service. To date there is no literature describing gender-related differences of NNRTIs.
In this study the investigators collected retrospective data obtained from 100 women and 268 men receiving NVP and 38 women and 156 men receiving EFV. Samples were included for patients using 200mg NVP BID and 600mg EFV QD and information on gender, age, weight, indication for TDM, co-medications, plasma level and time between last dose and sampling was recorded. Toxic levels were described as >6.0 mg/L for NVP and >4.0 mg/L for EFV.
Differences in plasma levels were compared using a Mann Whitney test and Pearson Chi-Square and risk factors for the incidence of toxic drug levels were analysed by logistic regression.
NVP concentrations were significantly higher in women than men with a median NVP plasma concentration of 6.7 mg/L (IQR 4.9-7.9) in women vs 5.5mg/L (4.4-7.0) p=0.006. Unsurprisingly weight was also significantly lower in women than men 64kg (57.5-78.00) vs 77.2 (70.5-85.00) p<0.001. Women were also generally younger 35 (29-41) vs 44 (37-51) years. None of the other factors analysed were significantly different. Using their definitions 57% of the females compared with 40.7% of the males were classified as having “toxic levels”.
Almost identical findings were presented for EFV concentrations with median plasma concentrations in women being 3.0 mg/L (2.2-4.7) which were significantly higher than those in men 2.3 mg/L (1.5-3.4) p=0.03.
After ruling out patient weight using logistic regression analysis, the investigators cited gender alone to be the predicting factor for a toxic plasma level (p=0.02 for NVP and p=0.03 for EFV). They found no differences between genders, when considering the indication for TDM, co-medication and time between intake and sampling.
In summary, the investigators found both NVP and EFV levels to be higher in women than men in this cohort, which they speculate may in turn lead to greater incidence of drug toxicity. Dr La Porte noted that: “physicians should be alert to an increased risk for toxicity in females.”
Intracellular concentrations of 3TC-TP and AZT-TP are higher in women and may explain toxicity and antiviral response
Continuing the theme of gender and drug levels Peter Anderson from the University of Colorado presented an excellent paper on sex differences in intracellular triphosphate (TP) concentrations and intracellular dose response.
Currently nucleoside analogue reverse transcriptase inhibitors (NRTIs) are used as the backbone to virtually all antiretroviral regimens. Limited observational data has suggested that women may experience both stronger virological responses and greater toxicities related to NRTI use compared to men, but to date there has been no suggested explanation for these effects.
Unlike NNRTIs and PIs, NRTIs require intracellular phosphorylation to achieve their active state. Several papers presented at this meeting strengthened the observation that there is a very poor correlation between plasma levels of NRTIs and their intracellular active forms. However few studies have attempted to characterise the active forms of these drugs, predominantly due to the technical complications involved. The study summarised here is shortly to be published in AIDS. The premise of the study was that potent antiviral effects and excess toxicities sometimes observed in women starting HAART maybe related to the intracellular concentrations of the nucleoside triphosphates.
The patients enrolled were part of a concentration-controlled study of IDV, AZT and 3TC [Fletcher, AIDS]. Stored PBMCs and plasma were analysed for intracellular triphosphates and plasma concentrations respectively. ZDV and 3TC tri-phosphate concentrations were obtained from 33 subjects providing 310 samples. Analysis revealed that the half-life of AZT triphosphate and 3TC triphosphate were 7 and 22 hours respectively (which is in keeping with previous studies). Triphosphate levels were quantified using enzyme-linked immunoassay for ZDV and LC/MS/MS to measure 3TC. 2.3-fold higher triphosphate concentrations of ZDV were found in women (n=4, 42 samples) compared to men (n=29, 268 samples) p=0.002 and 1.6 fold higher concentrations of 3TC p=0.003.
Obviously a potential limitation of this study is the small sample size (particularly the number of women represented), and this finding requires confirmation in larger studies. Nevertheless it may provide a plausible explanation for the increase in toxicity sometimes reported in women using NRTIs.
Intracellular triphosphate concentrations are related to antiviral response
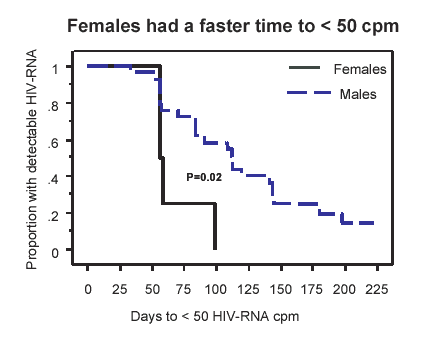
The same study also revealed another important effect of these concentrations – in answer to the question “were these higher triphosphates exerting any extra ARV potency?” it was found that time to reach <50 copies/mL was half the number of median days in women compared to men (p=0.02). Importantly the increased AZT-TP and 3TC TP was the only significant difference between the men and women shown in the table below:
| Males N=29 | Females N=4 | P (male vs female) Mann- Whitney | |
| Age (years) | 36 (30 to 42) | 33 (27 to 38) | 0.47 |
| Weight (kg) | 76 (56 to 112) | 68 (52 to 84) | 0.35 |
| Baseline HIV-RNA (log copies/ml) | 4.6 (4.2 to 5.0) | 4.6 (4.1 to 5.0) | 0.87 |
| ZDV Css (ng/mL) | 190 (164 to 216) | 210 (185 to 230) | 0.41 |
| ZDV-TP (fmol/106 cells) | 46 (30 to 67) | 106 (73 to 155) | 0.003 |
| ZDV Sample Times (hr post dose) | 2.8 (2.0 to 5.4) | 3.4 (2.0 to 5.8) | 0.68 |
| 3TC Css (ng/mL) | 473 (407 to 543) | 589 (491 to 689) | 0.12 |
| 3TC-TP (fmol/106 cells) | 8096 (6481 to 9801) | 12619 (10128 to 15852) | 0.002 |
| 3TC Sample Times (hr post dose) | 4.3 (2 to 7.8) | 3.0 (2.0 to 5.2) | 0.55 |
| IDV Cmin (ng/mL) | 130 (90 to 160) | 120 (90 to 155) | 0.85 |
(Table and slide courtesy of P Anderson unpublished data)
References:
- La Porte C, van der Ende M, Gyssens I et al. Gender differences in nevirapine and efavirenz pharmacokinetics. 4th Intl Workshop on Clinical Pharmacology of HIV Therapy, Cannes 27th-29 March 2003. Abs 10: P 3.1 and Abs 15: P 3.6
- Burger D, Siebers M, Hugen PWH et al. Pharmacokinetic variability caused by gender: do women have higher indinavir exposure than men? 2nd Intl Workshop on Clinical Pharmacology of HIV Therapy Noordwijk, The Netherlands 2-4 April Abs 4.2
- Burger D, Muller RJ, van de Leur MR et al. Lopinavir plasma levels are significantly higher in female than in male HIV-1 infected patients. 2nd Intl Workshop on Clinical Pharmacology of HIV Therapy. Washington DC,USA 11-13 April. Abs 6.5
- Anderson P, Kakuda T, Kawle SP et al .Sex differences in zidovudine (ZDV) and lamivudine (3TC) triphosphate concentrations in HIV-infected patients. 4th Intl Workshop on Clinical Pharmacology of HIV Therapy, Cannes 27-29 March 2003. Abs 53 P 7.2
Comment
It is strange in the nevirapine/efavirenz study that there was no difference in co-medications as one might, for example, expect some oral contraceptive use among women.
In the NNRTI study many in the audience felt the gender difference might simply be explained by body weight (an important finding in its own right) but in the logistic regression model presented, gender was the single predicting factor for potentially toxic NVP levels whereas age and body weight were not. Despite the limitations the potential effect of gender on NNRTI concentrations and toxicity may turn out to be important.
Several areas within this research require clarification in future trials, not least the therapeutic ranges used within these studies. In fact, one of the major rate limiting steps in establishing effective TDM services is setting biologically relevant cut offs for both efficacy and/or toxicity. Currently these are based on a small amount of published data and expert opinion. [refs Acosta , ARHR, BACK AIDS]. For example the so-called “toxic threshold in this study” for NVP is 2,000ng lower than the French ANRS guidelines.
The effect of long term high drug concentrations in virologically suppressed individuals is currently unknown. However, like the protease inhibitors, higher drug concentrations are probably required in drug-experienced populations than in drug naïve patients. Therefore maintaining the balance between virological efficacy and minimizing toxicity may be difficult in more experienced patients.
In the intracellular study, triphosphate concentrations were deemed an independent predictor of response even when the baseline viral load was controlled for, although It is possible that being female or having high intracellular triphosphate concentrations may be associated with another as yet unmeasured mechanism associated with beneficial response.
This is the first report of a relationship between intracellular drug concentrations and antiviral effect. This obviously opens the door to potential therapeutic interventions.

