The tuberculosis diagnostic pipeline
14 July 2011. Related: Pipeline report, TB coinfection.
Javid Syed
Introduction
After a spurt of activity in new tuberculosis (TB) diagnostics and algorithms approved for widespread use by the World Health Organization (WHO), this year the TB diagnostics cupboard is bare, with no new test or strategy expected to be reviewed for WHO approval until next year.
This lull is due to the dearth of investment in new TB diagnostics, despite the still pressing need for a true TB point-of-care (POC) test.
However the TB and TB/HIV programme implementers of the world should be busy this year with the important rollout and scale-up of the Xpert MTB/RIF TB test in high-burden countries where the new test – which can diagnose TB and multidrug-resistant tuberculosis (MDR-TB) within two hours rather than the two months of traditional culture – must rapidly be implemented if its lifesaving promise is to be realised.
This 2011 TB diagnostics pipeline focuses on products or strategies likely to be submitted for review by the WHO for use worldwide in the next three years.
From 2007 to 2010, the WHO’s Strategic and Technical Advisory Group for Tuberculosis (STAG-TB) reviewed and recommended the widespread implementation of eight new diagnostic tests and strategies (see Table 1).
Table 1. Diagnostic tests approved by the WHO, 2007?2010*
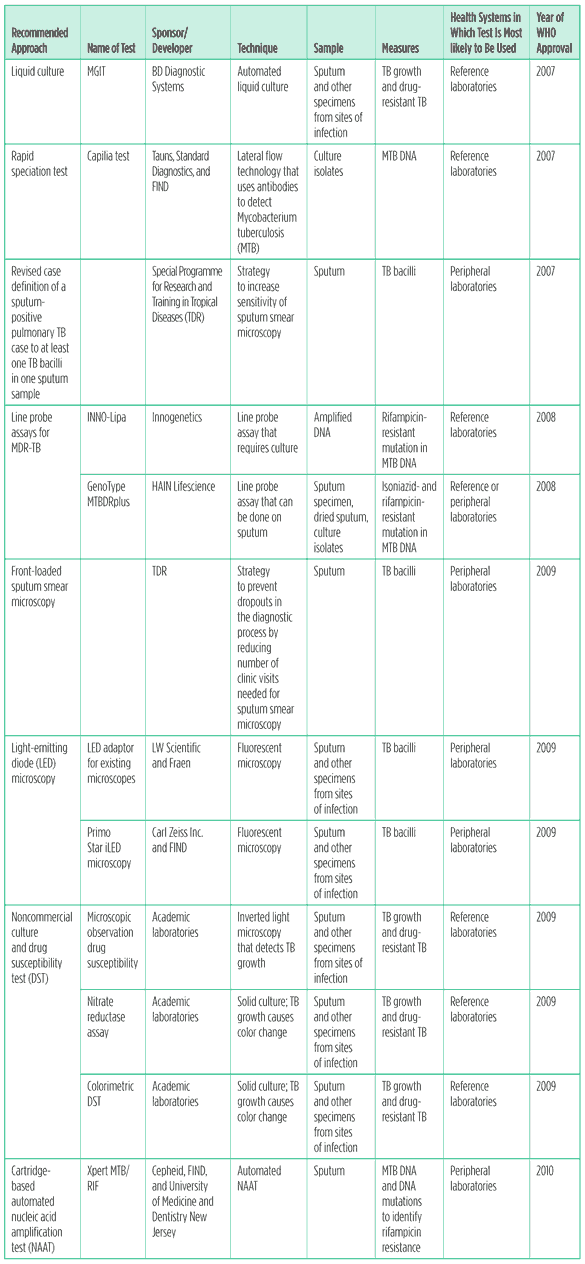
Sources: World Health Organization 2007b, 2008b, 2009, and 2010b.
These new tools have the potential to vastly speed up diagnosis and permit prompt initiation of proper treatment for TB. They could bring previously inaccessible technologies closer to patients, improve the accuracy of testing available at peripheral district health centers, and increase the speed with which TB and drug-resistant disease can be confirmed at both peripheral and reference laboratories.
However, none of the newer TB tests is ideal because of the cost, complexity, requirements for electricity, laboratory infrastructure, biosafety equipment, and the need for highly trained staff. The rollout of these newly recommended technologies will be slow and riddled with challenges. No TB test yet recommended by WHO is a true POC test appropriate for health posts – where 60% of people with TB seek services – or household testing (O’Brien 2009).
The lack of scientific investment is the key barrier in TB diagnostics discovery and development. To restore the TB diagnostics pipeline to health, robust, sensitive, and specific biomarkers for MTB infection, disease, and cure need to be discovered and technology platforms developed that can detect them.
Factors essential in a new TB diagnostic
The utility of a test is defined by the following factors linked to test accuracy and the place within the health system that the test is likely to be used.
Sensitivity: The ability of the test to accurately identify people with the disease. Low sensitivity of a test will cause people who have the disease to not be identified, not get appropriate treatment, suffer due to disease progression, and transmit the disease to others.
Specificity: The ability of the test to accurately identify people who do not have the disease. Low specificity means that more people who do not have a disease will wrongly be identified as having it, leading to inappropriate treatment.
Impact of test results on clinical decisions and patient outcomes: Sensitivity and specificity are surrogates for a test’s ability to improve treatment outcomes. Even a highly sensitive and specific test may not result in improved treatment decisions or reduce morbidity and mortality if it takes too long to provide results, thus failing to allow prompt initiation of proper treatment (Stall 2011).
Diagnostic algorithm: An algorithm is a recommended sequence in which tests and procedures – such as symptom screens – can be used for diagnosis and treatment. Even a less-than-perfect test can improve access to treatment depending on how it can be paired with other diagnostic tools in an algorithm.
Where within the health system the test can be used: A test’s usefulness depends in part on how decentralised its use can be. In this report the health system is divided into the health posts, peripheral laboratories, and reference laboratories.
- Health posts: These are the most decentralised locations of the health system, serving 60% of TB patients. They do not have access to electricity, water, or trained laboratory staff, and do not support diagnostic or biosafety equipment.
- Peripheral laboratories or health centers: These settings include district hospitals and laboratories and serve 25% of people in need of TB services. They have trained staff and the capacity to conduct sputum smear microscopy but only inconsistent electricity and minimal biosafety capacity
- Reference laboratories: These sophisticated laboratories serve 15% of those in need of TB services. They have highly skilled staff, reliable electricity and water supply, can ensure biosafety, and can conduct culture and nucleic acid amplication tests (NAATs) (O’Brien 2009).
Table 2. TB diagnostic tests and processes in the pipeline, 2011
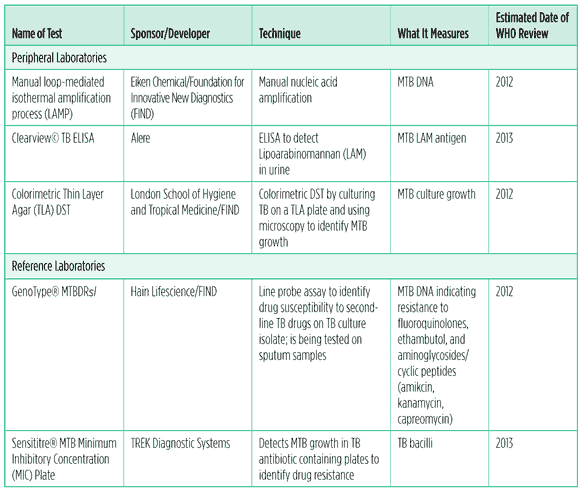
What is in the TB diagnostic pipeline?
At the heath post level
An instrument- and electricity-free POC or lateral flow dipstick test with simple training and no biosafety requirements would be ideal for the health post level. A quick, accurate, noninvasive POC test that allows for same-day results could have a massive impact on early case detection and appropriate treatment initiation. Immediate initiation of treatment would reduce onward transmission of TB (Abu-Raddad 2009; Medecins Sans Frontiers/Treatment Action Group/Partners in Health 2009). Several research avenues are being pursued to reach this elusive POC dipstick test. Volatile organic compounds from the breaths of people with active TB disease are being analyzed using modern technology like gas chromatograph/mass spectroscopy or through less high-tech methods such as using the giant African pouch rat to essentially smell breath and detect TB (Phillips 2010; Poling 2010; Weetjens 2009).
The Foundation for Innovative New Diagnostics (FIND) has identified TB proteins making up 0.5% of the total TB genome that are reactive to serum from persons with active disease. Lead candidate proteins that could be useful for a POC test are being purified (Kunnath-Velayudhan 2010).
Alere is developing DetermineTM TB LAM Ag, a lateral flow test for use in health post settings. Similar to Alere’s Clearview TB LAM ELISA test, the Determine test detects lipoarabinomannan (LAM) in urine. LAM is a TB cell wall protein released by metabolically active TB bacteria. The noninvasive test detects TB in unprocessed urine samples, making sample collection easier than sputum collection. Like the Clearview, the Determine assay may be more sensitive in people with HIV with advanced immunodeficiency, a population in which TB is currently hard to diagnose. Alere states that the test can provide results in 25 minutes. Studies are evaluating the usefulness of this test for diagnosing extrapulmonary TB and TB in HIV-positive children (Baker 2011).
No POC test is likely to be ready for STAG-TB consideration in the next three years.
In peripheral laboratories
1. The LAMP TB assay
A loop-mediated isothermal amplification process (LAMP)-based NAAT by Eiken Chemical and FIND is being studied for use in peripheral laboratories. If validated, this manual NAAT could replace microscopy.This closed-system LAMP test does not require heating and cooling, highly trained laboratory workers, or advanced biosafety equipment, as the sputum processing and instrumentation required have been greatly simplified. The prototype is being studied in Japan and elsewhere. The previous version of the test had high sensitivity (97.7%) in smear-positive sputum specimens, but only 48.8% in smear-negative sputum, while the specificity was high at 99% in both (Boehme 2007). No data are available on the sensitivity and specificity of the new prototype. The LAMP test may go before STAG-TB in 2012.
2. The LAM TB ELISA test
Alere’s Clearview TB ELISA test detects LAM protein in urine within three hours, using antibodies that bind with LAM in the urine sample. The bound antigens cause a color change to indicate presence of TB.
The LAM ELISA test was 59% sensitive and 96% specific in one study. The test sensitivity increased as CD4 cell counts declined in HIV-positive people and was 85% in those with CD4 cell counts below 50 (Shah 2009). This inverse relation between LAM ELISA’s sensitivity and CD4 cell count was seen in another study where test sensitivity reached 67% in those with fewer than 50 CD4 cells, but the sensitivity in people with CD4 cell counts higher than 100 was very low at 4% (Lawn 2009). The quantitative analysis of LAM using the Clearview test showed that a higher measure of LAM correlated with greater bacterial burden in sputum, with disseminated TB in the blood of HIV-positive people, and with lower CD4 counts (Shah 2010). A study in South Africa is comparing the utility of both Alere’s LAM Clearview ELISA and its Determine LAM lateral flow tests to diagnose TB among people with HIV initiating antiretroviral therapy. Clearview TB ELISA is available commercially in the United States and may be brought to STAG-TB in 2013 (Baker 2011).
A meta-analysis of results obtained with the LAM ELISA test confirmed that though this noninvasive LAM assay may identify TB in HIV-positive people with severe immune suppression, its suboptimal sensitivity requires that the populations in which it can be used be defined carefully (Minion 2011).
3. Rapid colorimetric drug susceptibility testing
FIND and the London School of Hygiene and Tropical Medicine are studying the feasibility of a rapid colorimetric drug susceptibility testing (DST) method using thin layer agar (TLA) at microscopy centers. Colorimetric DST is conducted by culturing TB from sputum sample on a TLA plate that has four different colored quadrants, three of which contain isoniazid, rifampicin, and a quinolone. The plate is sealed after the sputum is transferred onto the plate, which obviates the need for rigorous biosafety precautions. The plate is then incubated; any growth causing a color change is examined under a microscope to confirm MTB. Results take two to three weeks (Sandarac 2011).
A systematic review and meta-analysis of three colorimetric DST studies using non-TLA methods showed that the method was 100% accurate for detecting rifampicin and isoniazid resistance, and the mean time to result was 11 days (Minion 2010). The TLA DST’s sensitivity and specificity needs to be confirmed.
In reference laboratories
1. The GenoType® MTBDRs/
The WHO approved line probe assays (LPAs) for rapid diagnoses of MDR-TB in 2008. MDR-TB is resistant to two of the most powerful first-line TB drugs, rifampicin and isoniazid. The MTBDRsl test by Hain Lifescience is an LPA being developed to detect certain second-line TB drug resistance-associated genetic mutations. The test uses probes to detect gene mutations associated with resistance to fluoroquinolones (FQs); aminoglycosides/cyclic peptides including the injectable TB drugs amikacin, kanamycin, and capreomycin; and the first-line drug ethambutol. When used with the LPA for rifampicin and INH resistance, this test can identify extensively drug-resistant TB (XDR-TB) strains – MDR-TB strains also resistant to any FQ and at least one second-line injectable.
Peer-reviewed studies examined the test’s ability to detect resistance to mutations in clinical isolates, but not on direct sputum samples. One such study showed the test to be sensitive in detecting FQ (75.6%) and kanamycin (100%) resistance and less so for ethambutol (64.2%). The specificity was 100% for all three drug classes (Kite 2010). Similar results from another study showed MTBDRsl sensitivity and specificity to be 87% and 96% for FQ, 100% and 100% for amikacin, 77% and 100% for kanamycin, 80% and 98% for capreomycin, and 57% and 92% for ethambutol respectively (Brosier 2010).
The rapid LPA to detect XDR-TB provides results in less than five hours, and can prevent inappropriate treatment with ineffective drugs to which the patient will not respond. In 2010 a WHO-convened expert group considered the MTBDRsl test but a decision on the test was postponed until further data are available on its performance using direct sputum samples. FIND and Hain Lifescience are gathering these data. STAG-TB may review the results in 2012.
2. The MTB MIC plate
The US National Institutes of Health?funded TB Clinical Diagnostics Research Consortium (CDRC) and TREK Diagnostic Systems are collaborating to study the Sensititre® MTB MIC Plate. This test contains 12 first- and second-line anti-TB drugs in a single plate for the assessment of minimal inhibitory concentrations (MICs). The plate has a minimum of seven dilutions for each of 12 drugs. To detect drug-resistant TB the plates are inoculated with the TB isolates, sealed, and then incubated at 34?36 degrees Celsius for up to 21 days. Resistant strains are usually detected in ten days. The plate eliminates the need for each laboratory to create its own antibiotic dilutions as it contains standardised quality assured antibiotics at correct concentrations. This can avoid a major source of errors in conducting DST. The CDRC is studying the Sensititre MTB MIC Plate to assess the incremental improvement it can offer in identifying multidrug?resistant and extensively drug-resistant TB. WHO may review the results by 2013 (Dorman 2011; Sullivan 2010; TREK Diagnostic Systems 2011).
Recommendations
Resources for a point-of-care test
The biggest unmet need in TB diagnostics is for a POC test. There is an urgent obligation to fully resource efforts to identify biomarkers that can detect those at risk for progression from TB infection to active disease, and biomarkers correlated with disease, cure, and drug resistance.
To accelerate this progress, in February 2011 the Bill and Melinda Gates Foundation announced a new grant programme, Biomarkers for the Diagnosis of Tuberculosis, which will provide up to US$12 million to identify host and/or pathogen biomarkers that can quickly identify TB disease in low-resource settings.
Biomarker discovery requires well-characterised samples from people with and without TB and at various stages of disease and cure so that candidates can be validated in specimens from a wide variety of patients. In 2010 the US Food and Drug Administration provided funds to the TB Alliance to create a sample bank with the TB Trials Consortium and the AIDS Clinical Trials Group; the Consortium for TB Biomarkers (CBT2) will bank samples collected from study participants, many of whom will be followed through the duration of the study and monitored for relapse. It will have well-characterised samples from different phases of TB disease and cure. Although this effort is focused on identifying surrogate biomarkers for treatment outcomes, the CBT2 may provide opportunities for biomarker discovery work.
As the CBT2 is being established, another sample bank at the WHO’s Special Programme for Research and Training in Tropical Diseases (TDR) is in danger of being closed due to budget cuts. Instead of closing, sample banks need to expand and store a wider variety of samples in order to support the research required for the development of new diagnostics.
Ensuring that the global TB diagnostics pipeline includes all developers
The current diagnostics pipeline of the New Diagnostics Working Group (NDWG) of the Stop TB Partnership does not include all TB diagnostics in development. There is an urgent need for the NDWG to conduct an independent and transparent assessment of TB diagnostics in the all stages of development using agreed-upon specifications. The WHO and the Stop TB Partnership need to facilitate new developers and funders entering the TB diagnostics field. The WHO should clarify data standards it requires a product to meet to pass an expert review and be recommended by STAG-TB.
Addressing regulatory gaps in TB diagnostics
TB diagnostics are not well regulated, especially in high-TB-burden settings. This is demonstrated by the fact that commercial serological tests for TB antibody detection are available in 17 of 22 high-TB-burden countries, despite evidence of their poor performance and though no international guideline recommends their use. In India alone it is estimated that 1.5 million serological tests were done annually at a conservative cost estimate of US$15 million – most of which was borne by patients (Gernier 2011). The TDR conducted an evaluation of the performance of 19 commercially available rapid antibody detection tests for the diagnosis of TB and found that the sensitivity of all the tests was very low, the highest being 59.7% (World Health Organization 2008a). Based on these data the STAG-TB passed a negative recommendation against the use of commercial serological tests for TB in 2010 (Morris 2011; Steingart 2011; World Health Organization 2010b).
In 2010 the WHO recommended against the use of interferon gamma release assays to diagnose active or latent TB in low- and middle-income countries (World Health Organization 2010b).
Information about the WHO-recommended TB diagnostic tests and algorithms should be widely disseminated to educate all TB providers and civil society organizations to promote proper use of good tests and procedures and to prevent inappropriate use of inaccurate diagnostics (Specter 2010). Regulation of diagnostics in high-burden countries needs to be improved and incentives are needed to encourage the private sector to replace serological tests with WHO-endorsed tools (Pai 2011).
Scaling up WHO-recommended diagnostics in the absence of better tests
Though an ideal TB diagnostic does not yet exist, the scale-up of recently recommended diagnostics could reduce disease burden among populations in greatest need for improved TB diagnostics.
1. Algorithms for the identification of smear-negative and extrapulmonary TB and to rule out active TB in HIV-positive adults at the peripheral laboratory level
The WHO has recommended several algorithms to diagnose and treat TB in people with HIV, a population in which TB is difficult to identify using the sputum smear test. There is growing evidence to support the scale-up of these algorithms for the identification of smear-negative and extrapulmonary TB in adults with HIV (World Health Organization 2007a, 2010a).
A South African study examined the use of the WHO algorithm to diagnose and provide rapid access to treatment for smear-negative TB in seriously ill people with HIV. The study showed that 83% of those whose access to treatment was managed with the WHO-recommended algorithm were alive eight weeks after admission compared to 68% of patients diagnosed and treated using standard practices (Holtz 2011). A study in Cambodia using the algorithm to diagnose TB in ambulatory HIV-positive people showed that the median time to treatment initiation was five days. The time to initiation was longest at nine days for smear-negative TB and shortest at two days for extrapulmonary TB. The sensitivity and specificity of the algorithm to diagnose smear-negative TB in people with HIV were 58.8% and 79.4%, respectively (Koole 2011).
A study that looked at the use of cough of any duration, fever, and night sweats to identify people with TB had concluded that the algorithm to screen out active TB should include a combination of all three symptoms rather than focus only on cough (Cain 2010). In 2010 the WHO developed a simple symptom-based screen to rule out risk of TB disease in people with HIV using current cough of any duration, night sweats, fever, or weight loss. A meta-analysis of observational studies had showed that using the absence of all of these four symptoms, the screen was able to accurately predict those without TB by 97.7% if the prevalence of TB in people with HIV was at 2%. The test’s ability to predict absence of disease declined to 90% when TB prevalence was 20%. Because of its ability to rule out the presence of TB disease this symptom screen is recommended to identify people with HIV who don?t have TB disease and can be given isoniazid preventative therapy (IPT) (Getahun 2011). Recent studies comparing 36 months of IPT to six months of IPT showed a 43% reduction of TB in people with HIV living in Botswana (Samandari 2011).
2. Xpert® MTB/RIF: a recently recommended TB and MDR-TB diagnostic test for the peripheral laboratory level
In 2010, STAG-TB recommended the use of the Xpert MTB-RIF test – a rapid, automated NAAT that can diagnose TB and rifampicin resistance in two hours and does not require biosafety equipment or highly skilled laboratory workers. Because of its many advantageous specifications, the test can potentially be done at district health centers; however, it does need a consistent supply of electricity. Though it is shown to be cost-effective, its current price is US$17,000 for the machine and nearly US$17 per test (Roscigno 2010).These requirements will impede Xpert’s rollout in many settings. The Xpert MTB-RIF test’s overall sensitivity was 90.3% in all culture-confirmed TB and 76.9% in smear-negative cases. The specificity for the Xpert MTB-RIF test was 99%. For rifampicin resistance the Xpert test was 94.4% sensitive and 98.3% specific. The sensitivity of smear microscopy was lower than the Xpert MTB-RIF test at 67.1% and varied from 44.6% in people with HIV to 72.3% in people whose HIV status was negative or unknown.
Xpert MTB-RIF reduced time to TB detection to an average of zero days, compared with one day for microscopy, 30 days for solid culture, and 16 days for liquid culture. The time to initiate treatment for smear-negative TB was reduced from 56 days for smear-negative, culture-positive TB to five days for Xpert MTB-RIF. Although the Xpert MTB-RIF results did not inform initiation of MDR-TB treatment, the use of the test reduced time to detection of rifampicin resistance to one day, compared with 20 days for a line probe assay and 106 days for a culture DST. This study clarified the test could be run successfully on batteries; other factors like temperature and humidity still posed challenges. The manufacturer does not recommend the use of the test over 30 degrees Celsius, and the test cartridge stability requires the temperature to remain between 2 and 28 degrees Celsius. Dust and humidity caused a few breakdowns, but information was not available about the specifics of these failures and the subsequent cost or time to repair the machine. The company is devising methods to perform the annual calibration of the machine in the least disruptive manner (Boehme 2011).
Since the first evaluation of Xpert MTB-RIF in 2010, at least 15 studies and articles have been published. The accumulating evidence clearly shows that the test outperforms sputum smears in everyone and especially in HIV-infected persons, and it reduces time to results and allows more rapid initiation of treatment. Studies are needed to examine its usefulness outside a district laboratory setting and examine its effect on improving treatment outcomes.
Disseminating results of diagnostics scale-up to strengthen TB diagnostics advocacy
The WHO and the Stop TB Partnership should work with the lead sponsor of a recommended diagnostic to track the rollout of recommended tools, facilitate coordination of its implementation, and gather operational and outcome data important to patients and programmes. Documenting the lessons and successes of scale-up can inform advocacy for increased funding and highlight effective practices to expedite scale up effective diagnostics.
The WHO’s Global Laboratory Initiative is creating a website to provide information on the uptake of the Xpert MTB-RIF test. This website will have data from partners implementing the test and track where it is being rolled out, who is providing funding, and how many tests are being performed. The website will collect information from the manufacturer to track challenges in scale-up and how these are being addressed.
Documenting the impact of effective diagnostics is essential to support advocacy to increase global funding for TB diagnostics, which in 2009 was at a paltry US$41 million – far short of the US$740 million need estimated by the Global Plan to Stop TB: 2011?2015 ( Jim?z Salazar 2011).
Conclusion
After a burst of activity in the past four years, the pace of new diagnostics being brought to STAG-TB is slowing down, while there are major gaps in the availability of tools appropriate for use in all levels of the health system and especially at the health post level. Restoring a more robust pipeline requires a well-funded research agenda to develop biomarkers, technological platforms, and resources such as sample banks critical for the development of new diagnostics. Furthermore, a comprehensive and proactive strategy is needed to ensure that all diagnostic developers are contributing to the global TB diagnostics pipeline tracked by the NDWG.
References
Abu-Raddad LJ, Sabatelli L, Achterberg JT, et al. 2009. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA 2009;106(33):13980?85. Epub 2009 Aug 3.
Baker J 2011. Personal communication. 14 April 2011.
Boehme CC, Nabeta P, Henostroza G, et al. 2007. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol. 2007;45(6):1936?40.
Boehme CC, Nicol MP, Nabeta P, et al. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet 2011;377(9776):1495?1505. Epub 2011 Apr 18.
Brossier F, Veziris N, Aubry A, et al. 2010. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48(5):1683?89. Epub 2010 Mar 24.
Cain KP, McCarthy KD, Heilig CM, et al. 2010. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362(8):707?16.
Dorman S 2011. Personal communication. 19 April 2011.
Getahun H, Kittikraisak W, Heilig CM, et al. 2011. Development of a standardised screening rule for tuberculosis in people living with HIV in resource-constrained settings: Individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391.
Grenier J, Pinto L, Nair D, et al. 2011 Widespread use of serological tests for tuberculosis: data from 22 high-burden countries. European Respir J. 2011 (in press).
Holtz TH, Kabera G, Mthiyane T, et al. 2011 Use of a WHO-recommended algorithm to reduce mortality in seriously ill patients with HIV infection and smear-negative pulmonary tuberculosis in South Africa: An observational cohort study. Lancet Infect Dis. Epub ahead of print 2011 Apr 20.
Jim?z Salazar E 2011. Tuberculosis research and development: 2010 report on tuberculosis funding trends, 2005?2009. 2nd ed. New York: Treatment Action Group, 2011.
Kiet VS, Lan NT, An DD, et al. 2010. Evaluation of the MTBDRsl test for detection of second-line drug resistance in Mycobacterium tuberculosis. J Clin Microbiol. 2010;48(8):2934?39. Epub 2010 Jun 23.
Koole O, Thai S, Khun KE, et al. 2011. Evaluation of the 2007 WHO Guideline to Improve the Diagnosis of Tuberculosis in Ambulatory HIV-Positive Adults. PloS One 2011;6(4):e18502.
Lawn SD, Edwards DJ, Kranzer K, et al. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS 2009;23(14):1875?80.
Medecins Sans Frontiers/Treatment Action Group/Partners in Health 2009. Expert Meeting on Defining Test Specifications for a Point-of-Care TB Test. Paris, 17?18 March 2009. Retrieved 19 May 2011 from http://www.msfaccess.org/fileadmin/user_upload/diseases/tuberculosis/TB%20POC%20meeting%20outcomes%203.pdf.
Minion J, Leung E, Menzies D, et al. 2010. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10(10):688?98.
Minion J, Leung E, Talbot E, et al. 2011. Urinary lipoarabinomannan (LAM) antigen detection for the diagnosis of pulmonary tuberculosis: A systematic review and meta-analysis. Eur Resp J. 2011 (in press).
Moore DA, Caviedes L, Gilman RH, et al. 2006. Infrequent MODS TB culture cross-contamination in a high-burden resource-poor setting. Diagn Microbiol Infect Dis. 2006;56(1):35?43. Epub 2006 May 6.
Morris K 2011. WHO recommends against inaccurate tuberculosis tests. Lancet 2011;377(9760):113?14.
Nathanson CM, Cuevas LE, Cunningham J, et al. 2010. The TDR Tuberculosis Specimen Bank: a resource for diagnostic test developers. Int J Tuberc Lung Dis. 2010;14(11):1461?67.
O?Brien R. 2009 Progress in the development of new TB diagnostic tools – What is in the pipeline? Paper presented at the Fourth Scientific Symposium on the Occasion of World Tuberculosis Day, 22?23 March 2009, Berlin.
Pai M 2011. Improving TB diagnosis: Difference between knowing the path and walking the path. Expert Rev Mol Diagn. 2011;11(3):241?44. Phillips M, Basa-Dalay V, Bothamley G, et al. 2010. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 2010; 90(2):145-51. Epub 2010 Feb 26. doi:10.1016/ j.tube.2010.01.003.
Poling A, Weetjens BJ, Cox C, et al. 2010. Using giant African pouched rats to detect tuberculosis in human sputum samples: 2009 findings. Am J Trop Med Hyg. 2010;83(6):1308?10.
Roscigno G 2010. Xpert MTB/RIF: Update on price negotiations and market dynamics. Paper presented at the Implementation and Scale-up of the Xpert MTB/RIF System for Rapid Diagnosis of Tuberculosis and Multidrug Resistance Global Consultation, 30 November 2010, Geneva.
Samandari T, Agizew TB, Nyirenda S, et al. 2011. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: A randomised, double-blind, placebo-controlled trial. Lancet 2011;377(9777):1588?98.
Shah M, Martinson NA, Chaisson RE, et al. 2010. Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J Clin Microbiol. 2010;48(8):2972?74. Epub 2010 Jun 9.
Shah M, Variava E, Holmes CB, et al. 2009. Diagnostics accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalised patients in a high HIV prevalence setting. J AIDS 2009;52(2):145?51.
Kunnath-Velayudhan S, Salamon H, Wang H-Y, et al. 2010. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci USA 2010;107(33):14703?8.
Specter M 2010. A deadly misdiagnosis: Is it possible to save the millions of people who die from TB? New Yorker, 15 November 2010.
Stall N, Rubin T, Michael JS, et al. 2011. Does solid culture for tuberculosis influence clinical decision making in India? Int J Tuberc Lung Dis. 2011;15(5):641?46.
Steingart KR Flores L, Dendukuri N, et al. 2011. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: An updated systematic review and meta-analysis. PLoS Med. 2011 (in press).
Steingart KR, Ramsay A, Pai M 2007. Optimising sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev. Anti Infect Ther. 2007;5(3),327?31.
Sullivan N, Sotos J, Anhalt K, et al. 2010. Preliminary results from a new TREK Sensititre® Mycobacterium tuberculosis MIC plate (MYCOTB). Retrieved 19 May 2011 from http://www.trekds.com/techinfo/posters_abstracts/files/C-151.posterE.pdf.
Sundaram L 2011. Personal communication. 28 April 2011.
TREK Diagnostic Systems 2011. Sensititre® webpage. Retrieved 19 May 2011 from http://www.trekds.com/products/sensititre/c_ mycobacterium.asp.
Weetjens BJ, Mgode GF, Machang?u RS, et al. 2009. African pouched rats for the detection of pulmonary tuberculosis in sputum samples. Int J Tuberc Lung Dis. 2009;13(6):737?43.
World Health Organization 2010. Strategic and Technical Advisory Group for Tuberculosis (STAG-TB) Report of the tenth meeting. Geneva, Switzerland: World Health Organization, 2010. Retrieved 19 May 2011 from http://www.who.int/tb/advisory_bodies/stag_tb_report_2010.pdf.
World Health Organization 2008. Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis. Geneva, Switzerland: World Health Organization, 2008.

