Question
Q&As on COVID-19 vaccines: third doses, variants, chemsex, Halal?
2 July 2022. Related: All topics, COVID-19.
Answer
The following questions were for a community UK-CAB workshop on COVID vaccines. Answers by Angelina Namiba and Simon Collins. This page will be regularly updated with new questions and information. It was last updated on 2 Juty 2022 from an initial post on 22 December 2020.
Selected Q’s translated into Czech and into Russian.
Do HIV positive people need a third vaccine dose against COVID-19?
The detailed Q&A looks at latest issues around needing a third dose.
For most HIV positive people a third dose is not currently needed. But for a few people the third vaccine is important.
On 1 September 2021 the UK government included HIV in the health conditions for a third dose. This will mainly be for people with a CD4 count below 200 cells/mm3, but your HIV doctor can decide this.
What are the main COVID-19 variants? How can they affect vaccine responses?
This question is answered in detail at this link. It includes a table with ten major variants. This page is updated as new variants are reported.
Are vaccines against COVID-19 effective?
Yes, any approved vaccine has been very carefully studied in a wide range of people.
These first vaccines are highly effective. Both the Pfizer and Moderna vaccines prevent COVID-19 symptoms in about 95% of people. They also prevent severe COVID-19 and might help stop transmission.
The Oxford vaccine is at least 70% effective. However, comparing these top-line percentages doesn’t mean very much, This is because the studies were carried out at different times and in different people. They also defined efficacy in different ways.
All these vaccines are much better than first thought possible. At the start of the pandemic the target for an effective vaccine was set at 50%. All the current vaccines are much better than this.
Which vaccines are being used in the UK?
There are currently four COVID-19 vaccines that are authorised in the UK. Several others are expected during 2021.
On 28 May 2021 the UK approved a fourth vaccine (JNJ-78436725), made by Janssen/Johnson & Johnson. It was approved in the EU on 11 March and in the US on 27 February. Although this vaccine only needs a single injection, it is not likely to be available in the UK until later in 2021.
The third vaccine that was approved in the UK is called BNT162b2 (tozinameran). The trade name is Comirnaty.
BNT162b2 is made by Pfizer/BioNTech. It was approved in the UK on 2 December, in the US on 12 December and in the EU on 21 December 2020.
On 8 January 2021, a second similar vaccine, developed by Moderna/NIH was approved in the UK. However, this is unlikely to be widely available until March/April 2021. The Moderna was approved in the US on 18 December 2020. The code name for this vaccine is mRNA-1273. It was approved in Europe on 6 January.
On 30 December 2020, the UK approved the vaccine from Oxford University and AstraZeneca. This is called ChAdOx1 (from Chimp Adenovirus Oxford). It also has the code name AZD1222.
Other vaccines are being used in UK studies (see later below). These include:
- A vaccine from Novavax that was studied in the UK and South Africa.
- A self-amplifying ribonucleic acid (saRNA) vaccine at UCL.
As new vaccines are approved we will add them to this page.
The PROVENT and STORM CHASER studies are looking at using a monoclonal antibody (AZD7442) for people who might not benefit from vaccines. GSK is also studying a monoclonal antibody (VIR-7831) in early infection.
Can I use the Indian COVAXIN vaccine if I am on ART?
Yes. This is part of the vaccination programme in India. The limited data seem good and given the high risks of COVID-19 any level of protection should be good.
A more detailed answer is at this link.
Why should I get a vaccine?
The main reason to get the vaccine is to protect yourself against COVID-19.
COVID-19 can be deadly – it is much better to be protected. Even people who recover from COVID-19 often have symptoms that last for many months. This is called long COVID and is still being studied.
If you have been offered the vaccine it is because of your personal level of risk. The vaccine may also protect your friends, family and contacts at work.
Is my risk high enough to need the vaccine?
There is only a limited supply of these vaccines.
In the UK, for at least the next few months, you will only be offered the vaccine if your personal risk is high.
This will be because of your age and your health or because you work in a high risk job.
Were HIV positive people included in COVID-19 vaccine studies?
Yes. People living with HIV were included in most vaccine studies. Usually these were small numbers though compared to the study overall.
This small group is still enough to compare immune responses to the general population. The main concern might be that the vaccines are slightly less effective.
There are NO safety concerns from these vaccines in people living with HIV. Or with any other common health conditions.
I already have one virus (HIV) – why would I want parts of another?
Okay, two answers to this one…
First, COVID vaccines have ZERO risk of giving you an active virus.
Second, our bodies already contain millions of different bacteria and thousands of viruses and fragments of previous infections. This is all normal and part of having a health immune system.
Our DNA goes back millions of years and includes fragments from tens of thousands of viral infections. (Related link)
Do I have to get the COVID-19 vaccine?
If the vaccine is for your own health, then this is always still your choice. You do not have to have the vaccine.
Please talk to your doctor if you have any worries or concerns. Or if you are not sure about having the vaccine.
If you are offered the vaccine because of your job, not having the vaccine might affect the work you can do.
In the future, some countries might want travellers to be vaccinated.
My HIV history included being told vaccines were a risk for me?
HIV positive people only have to avoid vaccines that contain live viruses.
Other vaccines are actively recommended for people living with HIV. These include vaccines for seasonal flu, pneumococcal infection, and now COVID-19.
Link about flu and pneumococcal vaccines during COVID-19.
Can I wait for a COVID-19 vaccine if I am young with a low risk?
Even if the average risk is low for your age, you can still benefit personally. Some young people had COVID-19 that lasted for many months and some have died.
If your history includes a low CD4 count, this might makes your risk from COVID-19 much higher. If you have been offered a vaccine, this is because you have a real risk from COVID-19.
Also, and really the main reason, is that a vaccine will protect other people who you see and live with. Having a vaccine is part of a wider community response.
Are COVID-19 vaccines free in the UK?
Yes. The NHS will not charge anyone for these vaccines.
ALL COVID vaccines are free in the UK. This includes whether linked to your health risk or to your job.
Never give out personal details linked to payment for a vaccine. This is a good sign of a scam.
Are vaccines against COVID-19 safe?
Yes, any authorised vaccine will also be very safe. We know this from the results of large studies, and from even wider use since.
For example, the Pfizer vaccine was first studied in more than 44,000 people. Since then, it has been used by tens of millions of people.
With vaccines, most side effects occur in the first six weeks. All approved vaccines will have studied thousands of people for at least this long. Often, any symptoms are very similar to the control or placebo group.
But within a few weeks of approval the Pfizer vaccine had been used by hundreds of thousands more. This is when reactions that are more rare can sometimes be reported.
In the UK this included two people who had a history of allergy reactions. This history was serious enough for them to always carry an adrenaline syringe. Both people were managed in hospital similar to other allergic reactions and they are both now well.
This led to only giving mRNA vaccines (Pfizer and Moderna) where there is medical support to treat allergic reactions.
People with a history of serious allergies should discuss the risks from an mRNA vaccine with their doctor. This includes to any of the listed ingredients in the vaccine, for example to polyethylene glycol. It is easy for even severe allergic reactions to be safely managed.
Other common allergies, including to medications, foods, inhalants, insects and latex, are not thought to increase allergic reactions to the mRNA vaccines. Please discuss the benefits vs risks with your doctor who knows your history.
In a similar way, shortly after the Moderna vaccine was approved in the US, there were several reports of reactions in people who had used facial fillers. This was in two people who had used facial cheek fillers within six months and one person who used lip filler two days after the vaccine.
All the reactions resolved after treatment with steroids and antihistamines. Responses to both Pfizer and Moderna vaccines in people who have used facial fillers should be prospectively tracked to understand how often similar reactions occur.
As facial fillers are commonly used to correct HIV-associated lipoatrophy, this might be a caution. In practice though, very few treatments are likely to have been available during 2020 because of COVID. Also, the main filler used in the UK for lipoatrophy (New-Fill) isn’t a permanent filler, but just regenerates collagen growth.
Other very rare reactions might still be reported. This is because even very large studies cannot include every type of medical history. Also, the studies mainly included people who were generally well and at low risk of COVID-19.
The seriousness of COVID-19 makes it much safer to have an effective vaccine even if rare reactions might happen. This includes for people with a history of allergies or past use of fillers.
It is probably not a good idea to use facial fillers immediately after a vaccine. Guidelines are likely to recommend this.
How do we know the vaccine is safe?
These vaccine are safe because studies showed there is a very low risk of serious side effects.
Compared to the very real risks from COVID-19, using the vaccine is much safer than not using it. This is known from research studies in tens of thousands of people. The studies recorded every side effect or any potential side effect.
Additional safety data comes after the vaccines are used outside of studies. This will include from people who were not included in the main studies. This led to a caution in people with history of serious allergic reactions (see next Q).
Does the Oxford/AZ vaccine increase the risk of blood clots?
The research on this question has been changing during March and April 2021. It relates to reports of very rare but serious blood clots in people who also had this vaccine.
Although the evidence for a link has slowly changed to support a very low risk, this is still being studied.
If the vaccine is the cause, the risk is much lower than 1 in 100,000 people.
Most cases were in women younger than 30 and UK guidelines continues to recommend general use. Women younger than 30 can use the alternative COVID vaccine from Pfizer.
What is the best/ideal interval between vaccine doses?
In the UK, NHS guidelines recommendations are slightly different for each vaccine.
- The second dose of the Pfizer/BioNTech vaccine can be given from 3 to 12 weeks after the first dose.
- The second dose of the AstraZeneca/Oxford vaccine can be given from 4 to 12 weeks after the first dose.
In practice, both vaccines are now being given with 12 weeks between doses. There is no choice for which vaccine you get or the timing of doses.
However, these recommendations have been made based on limited evidence, some of which is not publicly available. Pfizer, for example, only supports a three week dosing interval based on lack of evidence for anything longer, even though the NHS has extended this to 12 weeks. The limited information about the longer dosing of the Oxford vaccine came from the UK study using the half-dose (which is not being given).
Individual protection will be better when the second dose is given earlier – i.e. after either 3 or 4 weeks. This is because full protection will be reached more quickly.
On a population level, government policy is to try to give the first dose to as many people as possible. This is looking at reducing risk for the UK as a whole given limited access to vaccines. The policy also recommends that the same vaccine should be used for both doses.
The first dose of either vaccine will give a good level of protection. Other vaccines have shown that giving the second dose later can be better than giving it too early.
The real answer is that there is not enough evidence to answer this question.
For more information please see: NHS letter on vaccine roll-out (PDF)
Am I protected after the first dose of a COVID-19 vaccine?
All the current vaccines need to be given in two doses.
In the UK, the second dose is now being given after 12 weeks. This means that full protection doesn’t develop until a few weeks after the second dose.
Although you get some protection at the first dose there have been lots of examples where people still had COVID-19.
Please continue to limit your risk of catching or transmitting COVID-19 before this. Please continue to wear a mask in shared indoor spaces, to limit social distancing and to use hand hygiene until you have the full protection from the vaccine.
Some guidelines still recommend being careful after this time.
Will the COVID-19 vaccines work against the new variant viruses?
Some researchers think the vaccines will still be effective against new variants.
This includes the recent variations that were first reported in the UK, South Africa and Brazil. However, this is still the subject of intense research.
Many viruses make small changes over time. This is just part of how viruses evolve. Although most changes are not important, they can sometimes make the virus easier to transmit.
This recent study also says that mRNA vaccines should be easy to modify if more difficult strains develop.
See these links for more detailed information:
- What are the main COVID-19 variants and will they affect vaccines?
- Pfizer COVID-19 vaccine might still overcome UK and SA variants.2
Can I mix doses with different COVID-19 vaccines?
This is a good question that might change in the future, based on new data.
Currently, UK recommendations are to use both the same vaccine for both doses.
However, there are rare times when different vaccines can be used. These include:
- When protection from the second vaccine is urgent if same vaccine is not available.
- If there is no record of the make of the first vaccine.
- In these cases, any second vaccine would be better than missing the second dose.
- This is NOT a recommendation to routinely mix vaccines.
This is based on guidance in the UK Green Book, chapter 14a (PDF)
Can I take other vaccines at the same time as COVID-19 shots?
If you need to have other vaccines if is best to separate them by about a week.
Have the most important vaccine first – which for mot people with be COVID-19.
Having other vaccines a week later with make it easier to know which one causes any side effects.
For example, if you need flu, pneumococcal or hepatitis B vaccines too.
If the HepB vaccine didn’t work for me will the COVID vaccine work?
Expert advice is that the COVID vaccine response is unlikely to be linked to other vaccines.
Although this hasn’t been studied or reported yet, but it is better to still try for COVID protection.
Even if the vaccine cover is not quite as good, this will still have some benefit. At some point, researchers might recommend some people have an additional shot – but this will be based on future research.
Responses might be reduced if your CD4 count is currently very low (less than 50 cells/mm3). Or if it was ever this low in the past.
Can I use an antibody test to check if the COVID vaccine worked?
Antibody tests for diagnosis are different to antibody tests for vaccine response.
They test for different types of antibodies. So you can have full vaccine protection and still test negative on a diagnostic test.
Guidelines currently do not recommend testing for individual responses to the COVID vaccine. This is even in people who might be expected to have a lower response.
Even using the right test, scientists have not yet worked out a target level for immune responses to be protective.
They specifically advise not to used antibody tests as these are not likely to produce reliable results.
The negative result does not mean the vaccine did not work. The immune system is complex, and this test is not sensitive enough to show if you have protection.
What is in the COVID-19 vaccines?
None of the COVID vaccines in the UK contain live viruses. There is no risk of catching coronavirus from the vaccine.
The active parts of a vaccine only use a protein from the outside of the coronavirus. Or they tell your body how to make these proteins.
This does not cause an infection.
Vaccines also include other ingredients that help the vaccine work. For example the Pfizer vaccine contains traces of sodium and potassium. This is sufficiently low to still be called sodium-free and potassium-free.
It also contains sucrose and this, together with all other ingredients, is listed on the patient leaflet that you get before the injection. This is also online now if you want to check first (see further information in the final question linked below).
Are animal products in the vaccines? Are they Halal and Kosher?
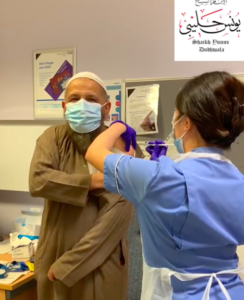
- There are no animal products in the Pfizer, Moderna or Oxford vaccines.
- There are also no traces of pig products or egg products.
- These vaccines are all Halal safe and Kosher.
This link to a two-minute clip from Imam Yunus having the vaccine at Newham Hospital in East London.
As chaplain of St Barts, Imam Yunus talks about how the vaccine is safe, effective and Halal:
Video – Imam Yunus on how the vaccines are safe if you are Muslim.
Please note this video is included as an information resource. It is not directly liked to HIV and i-Base was not involved in producing it.
What if I have a history of allergy reactions?
As in the question above, even people with a history of serious reactions can still use the vaccine. This includes people who have reactions to vaccines, medicines or foods.
However, if you currently need to carry an anti-allergy syringe, you need to be vaccinated in a clinic in case a reaction occurs.
Two health workers in the UK with a history of severe reactions did react to the Pfizer vaccine. Both people have now recovered. More information will be collected on cases like this.
Can I develop an allergic reaction to COVID-19 vaccines?
Reactions to vaccines are rare but they have been reported with some COVID-19 vaccines. The risk relates to your history of allergies but you can still use the vaccines.
Both the mRNA vaccines (from Pfizer and Moderna) should only be given in a setting with medical support to be able to handle allergic reactions.
Anyone with a history of severe allergy reactions can still use these vaccines. But they should discuss the risk/benefits with their doctor.
However, anyone who has a severe reaction to the first dose of these vaccines, should not take the second dose.
Guidance from the US on risk of allergic reactions to mRNA COVID-19 vaccines
What about if I have immune suppression from HIV or cancer treatment?
Yes, the vaccine is still recommended if you are HIV positive or if you have cancer. This is because of the high risk from COVID-19.
Although the leaflet that comes with the Pfizer vaccine includes talking to your doctor first if you have a reduced immune system, this is not related to a safety concern with the vaccine. It is only because the protection from the vaccine might not be as strong.
This means that even after both doses of the vaccine, it might still be important to be careful. For example, if the pandemic is still at a high level, by wearing a mask and social distancing. But this is similar to advice to the general population.
As more people are vaccinated, researchers will look at responses in people who were not widely included in studies. It is worth being cautious until we have these results. They might also find that responses are just as strong in people living with HIV or cancer.
What if I have other inflammatory or autoimmune conditions?
As above, the vaccine is still recommended for people living with inflammatory or autoimmune conditions. This includes people using immune suppressing drugs.
In this, it is very similar to getting a flu vaccine. Anyone who can use the flu vaccine can use a vaccine against COVID-19.
These include:
- Inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus).
- Inflammatory bowel disease (Crohn’s disease and ulcerative colitis).
- Psoriasis.
- Multiple sclerosis.
- Organ transplant recipients.
- People on chemotherapy.
This is because of the high risks from COVID-19.
Although only a subset of people with these and other complications are so far included in vaccine studies, there is no safety concern. As above, the caution is that the vaccine might not be quite as effective.
Ongoing research though will be looking at this but this article is also helpful:
Why vaccines are recommended for people with immune suppression and autoimmune conditions
Can people with haemophilia and HIV have COVID-19 vaccines? Are there special cautions?
Yes, the COVID-19 vaccines are recommended for people with HIV and haemophilia. However, many people will need clotting factor treatment immediately before they have the injection. This is because, although rare, people with haemophilia can develop serious bleeding after an intramuscular injection.
There are no problems with the vaccine, just a caution over a potential problem linked to an injection.
The COVID-19 vaccine must be given as an injection into the muscle in your arm. This is even though some vaccines like the flu vaccine can be given either into the muscle or under the skin.
People with haemophilia should only be given the vaccine “if their haemophilia doctor thinks it can be done safely”. This is likely to be true for probably everyone with haemophilia and HIV in the UK. As stated above, some will need factor treatment before having the vaccine.
What extra precautions should people with haemophilia and HIV take for the COVID-19 vaccine?
The following suggestions include:
- Use a small size needle for the vaccine injection (23 or 25 gauge is recommended in the UK).
- People with haemophilia and HIV who regularly take clotting factor treatment will need to have treatment to prevent any bleeding just before the vaccine injections.
- Firm pressure should be applied to the injection site for two minutes afterwards.
- Some people with moderate haemophilia will not need any treatment.
- People with haemophilia and HIV who are taking emicizumab should not need any extra treatment before being vaccinated.
What if I have haemophilia and inhibitors to a clotting factor?
If you have been on emicizumab for some time, you should be able to have the COVID vaccination injection without problems and no need of extra treatment.
Please check with your haemophilia centre if you are in this situation.
If you are still in the loading phase of emicizumab, your haemophilia doctor will need to decide what is needed to allow you to be vaccinated without causing any bleeding problems. The loading phase is usually the first 4-6 weeks.
If you are on another treatment, it is also important to check with your haemophilia doctor.
Where can I get more information about haemophilia and COVID-19 vaccines
The following resources include more information:
- COVID-19 vaccination programme: Information for healthcare practitioners (PDF -see page 16)
- COVID-19 vaccination guidance for people with bleeding disorders from the World Federation of Haemophilia (PDF)
- Green Book chapter 14a (PDF – see page 7).
Does the vaccine interact with other medicines?
No. There are no medicines that currently can not be used with the Pfizer and Moderna vaccines.
If you are taking other treatment, there is no need to stop this to have a vaccine.
Although it is good to ask about interactions with current medicines, there are no interactions with the vaccines. If you are worried, it is easy to double-check this with your doctor.
Your doctor will also know your medical history and whether one type of vaccine might be better for you than another.
Do I need to say I am HIV positive before getting a vaccine?
No. Injecting procedures are the same whether you are HIV positive or negative.
However, in the UK, your GP needs to know you are HIV positive in order to get earlier access.
Although one of the vaccine leaflets (for the Pfizer vaccine) does ask about HIV, you can ignore this if you want to. The leaflet is an old leaflet that is now out-of-date.
If you are asked to fill in a form about other medical conditions, you can also ignore HIV if you are on effective ART.
If you do include info on HIV, you might also be surprised. The person giving the vaccine might say “me too” or “yeah, my Mum is also positive”.
Why do some vaccine leaflets mention HIV?
Two of the vaccines includes information that refer to “immune suppression”. The Pfizer leaflet also includes HIV with this reference.
All these leaflets are out-of-date in this aspect of the information.
All vaccines are recommended for HIV positive people – just as the flu vaccine.
If the NHS leaflet asks about other health conditions, you do not have to mention HIV if you don’t want to.
Could the vaccine interact with my HIV meds?
There are no interactions between the COVID-19 vaccines and HIV meds.
Will my HIV viral load blip when I have the vaccine?
There is not enough results from HIV positive people in the first vaccine studies to report this yet, though this will be reported later.
Based on other vaccines, low-level blips might occur in a small percentage of people just after the vaccine.
These will not affect your HIV treatment or your risk to partners.
As with the answer to other questions here, it is okay to approach the COVID vaccination as if it was the annual flu vaccine – which is widely recommended for people living with HIV.
If your viral load is generally undetectable any increase is likely to be very small. For example, with the flu vaccine, it might increase from less than 50 to maybe 80 or 100 copies/mL – and only for a few days or a week. This is too low to affect the risk of transmission.
Can the vaccine interact with oestrogen and/or testosterone treatment?
There are no interactions between the COVID-19 vaccines and oestrogen and testosterone.
Are the vaccines safe in pregnancy?
Great question. So far there is little data because pregnancy was an exclusion for the main studies. But if you are pregnant, the vaccine is still recommended.
Also, women will still have become pregnant during these studies – and certainly afterwards. These data will all be collected during the study.
When these data are available they will be widely publicised.
Other studies are looking at vaccine responses during pregnancy.
Are the vaccines safe in children?
The first vaccine studies were in people who are aged 16 and over.
Studies in younger children are currently ongoing.
How are the COVID-19 vaccines given?
The vaccines used in the UK are given as an injection into your upper arm.
The Pfizer vaccine originally needed the second dose to be given three weeks later. The government later changed the second dose to be given after 12 weeks,
Although some protection starts after the first dose, full protection is not until seven days after the second dose.
The second dose of the Oxford vaccine is also given after approximately 12 weeks.
Do I still need to social distance after the vaccine?
This advice is going to change based on local risks and also as more data is collected.
During the first months of 2021, even after the vaccine, it might still be good to reduce the risk of catching coronavirus.
A few people might not be protected by the vaccine. We also don’t know how long protection will last – although it will hopefully be at least a year. If you still become infected without symptoms, you could then pass this to other people.
Please continue recommendations for wearing a mask and social distancing that is relevant for your area.
Can I get COVID-19 from the vaccine?
No. This is easy to answer.
There is zero risk of getting COVID-19 from the vaccine.
The vaccines do not contain live coronavirus that causes COVID-19.
If I get ill with COVID-19 will the vaccine help my recovery?
If you had the vaccine more than two weeks before COVID-19, then the vaccine will already be reducing your symptoms.
There is no data to know whether having the vaccine after getting COVID-19 will have any benefit.
What are the side effects from the vaccine?
Most side effects to the vaccines were mild or moderate.
The details below are from the Pfizer vaccine.
Very common side effects were similar to getting the flu vaccine. They generally got better within a few days. These were reported by more than 1 in 10 people.
- Mild pain at injection site (common).
- Tiredness.
- Headache.
- Muscle pain.
- Chills.
- Joint pain (uncommon).
- Fever (uncommon).
Common side effects included injection site swelling, redness at injection site, and nausea. These were reported in less than 1 in 10 people.
Uncommon side effects, in less than 1 in 100 people included enlarged lymph glands or just generally feeling unwell.
Swollen lymph glands in the arm and neck have been reported 2 to 4 days after the injection. These also only last a few days and will not affect your health.
Will I get sick with the COVID-19 vaccine like the flu jab?
No, not necessarily, but maybe. So far the COVID-19 vaccines are similar to getting a flu vaccine. And just like the flu vaccine, the response will vary for different people.
The question above shows that side effects are similar to the flu vaccine and are nearly always mild.
Can I still have the flu vaccine and how do I time this?
Yes. If you normally have the annual flu vaccine, you can still have this.
Getting the flu vaccine first might be easier if you are waiting for the COVID vaccine.
If you get offered the COVID vaccine first, then take up this offer and have the flu vaccine later. This should ideally be at least a week after the first dose. The order of these vaccines is not important.
Leaving at least one week between the two vaccines will help you know whether either of these gives you side effects.
Should I wait to see how people similar to me react first?
This is a good question – and sounds very reasonable. But within a week or two another 500,000 people will have used the vaccine in the UK.
Any serious concerns will be reported long before you are likely to be offered the vaccine.
If you want to interact with people, then waiting will be more risky than having the vaccine now. However, if you are okay leading a very isolated life, then waiting is a choice.
How long will protection from the COVID-19 vaccine last?
This will only be known with more time.
Protection should last for at least a year and hopefully a lot longer.
Some vaccines, for example hepatitis B and tetanus only need a boost every ten years. Other vaccines, like the flu, need to be given every year because the virus changes and adapts.
It is not clear yet how often coronavirus vaccines will be needed.
Which COVID vaccine is best?
So far, all the leading vaccines look very good. Getting access to any vaccine now is more important than which vaccine you use.
Once several vaccines are available, independent researchers will hopefully compare them with each other.
What if I already had COVID-19? Does it matter where this was severe or mild?
People who already had COVID-19 are still recommended to use the vaccine. It doesn’t matter how severe or mild this was.
The vaccine will already be used by many people who didn’t even know they have caught coronavirus before. And many people who had similar symptoms were not able to get tested.
Will my GP or HIV doctor give me the vaccine? Can I choose?
Who gives you the vaccine might depend on which vaccine is being used. It might be your GP or a hospital doctor. In the UK it is unlikely to be your HIV doctor.
The Pfizer vaccine will generally be given at health centres or hospitals. This is so any allergy can be easily managed. Also because of limits in how it can be stored.
The Oxford vaccines might be given by your GP because it is easier to transport and store. This is early stage for the vaccines but it is unlikely to be your HIV doctor.
However, if you get a letter from both a vaccine centre and your GP, you can chose which of these to use.
Please remember that all the current vaccines need to be given as two doses.
Why should I get the vaccine if the person giving me the vaccines hasn’t had it yet?
The decision on who gets the vaccine first is made by an expert advisory group. It is based on the risk of infection and the risk of severe disease.
If this group recommends you get the vaccine, then this is because your individual risk makes this important.
It is good that you are concerned about the health worker but they are also likely to get the vaccine soon.
Will the vaccine stop me catching COVID-19? Or just from getting ill? Or maybe both?
The vaccines will definitely reduce risk of getting ill, but the answer is “probably both”.
The vaccine are approved because they reduce symptoms of COVID-19.
The first studies didn’t measure whether people caught coronavirus, just whether they had symptoms of COVID-19. However, there is some evidence that the vaccines also protect against infection.
With the mRNA vaccines, most mild symptoms later confirmed as COVID-19 were in people who didn’t get the vaccine. Importantly, nearly all the most serious cases of COVID-19 were also in people who got the placebo (inactive) injections.
Technically, some people might still catch coronavirus and be infectious but without symptoms. This is still an ongoing research question.
Studies with the Moderna and Oxford vaccines include some results showing that the risk of catching coronavirus is also reduced.
Is the vaccine safe if I have other health problems as well as HIV?
Yes, vaccines are recommended in people living with HIV and other health problems.
The more serious your other health problems, the more important it will be to be protected from COVID-19.
Can I get the vaccine if I have or have had hepatitis C?
Yes, vaccines are recommended in people living with hepatitis C or who previously had hepC.
Is the vaccine safe if I use chems like crystal meth, GHB or mephedrone?
Yes, the vaccines do not interact with drugs used for chemsex.
However, taking a break from the chems for the week of the vaccine will make it easier to know whether you get any side effects.
If the social context for using chems means you are having more partners, the protection from the vaccine will be especially important.
Also, if your current situation or pattern of chem use makes it difficult to have a break for a week, it is still a good idea to get the COVID vaccine as soon as it is available.
Is the vaccine affected by ethnicity? Will it affect me differently because I’m black/brown?
No, vaccines studies include people of different ethnicities. They are created for everyone.
Ethnicity does not affect immune responses or risk of side effects with mRNA vaccines.
 This short video is from Nigerian GPs working in the UK who have all had COVID-19 vaccines. Nigerian GP video.
This short video is from Nigerian GPs working in the UK who have all had COVID-19 vaccines. Nigerian GP video.
Please note this video is included as an information resource. It is not directly liked to HIV and i-Base was not involved in producing it.
Are black and brown people more at risk of getting side effects from the vaccine?
No, as with the question above, ethnicity has not been linked to any better or worse outcomes after the vaccine.
Have vaccine trials included black and brown men and women living with HIV? Or do the findings just relate to the experiences of HIV positive white gay men?
Unfortunately, most vaccine studies only included very small numbers of people living with HIV. So far, the ethnicity breakdown of the HIV positive group has not been presented. All the HIV positive participants might be black and brown women.
For example, the Pfizer study with more than 44,000 people only included about 120 people living with HIV. The results did not show that HIV as any impact on how the vaccines work. Data though are currently limited.
However, there is more data about ethnicity – and more will come from the wider vaccine programmes.
About 10% of the people in the US sites for the Pfizer vaccine were black or African American. There were no differences in how well the vaccine worked or in side effects compared to the rest of the study population.
In the Oxford vaccine studies about 90% and 65% of participants were white in the UK and Brazil sites respectively. Although there were very few black participants in the UK sites this was around 10% in Brazil.
Who approved these vaccines? Were the interests of my community represented?
Vaccines are approved by the same organisations that approve medicines. They were approved for all people.
- This is the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK.
- In Europe it is the European Medicines Agency (EMA)
- In the US it is the Food and Drug Administration (FDA).
- Other countries and regions have similar organisations.
Each of these groups is made up of expert advisors who are mainly scientists and doctors. Sometime they include community voices.
The panels are responsible for representing interests of all people who are going to be using these products.
The FDA publishes the detailed study results online for everyone to read. If also webcasts the meeting that decide if a vaccine or medicine will be approved.
How do I know I’m being treated equally? How do I know this isn’t experimentation in black people?
These concerns about equality are very real.
Nearly all countries still have structures that are not equal. Many have a history where people were treated differently.
In the UK, this still affects access to important services that include education and medical care. This is even when there are policies to make access fair.
However, ethnicity has been linked to higher risk of COVID-19 in black, Asian and minority ethnic (BAME) communities. This actually makes access to the vaccines even more important.
As all the studies included people from all ethnicities. There is good data to show they are at least as safe and effective.
COVID vaccines will be offered to people of all ethnicities. As has been seen in the news all ethnicities have the choice to use the vaccine.
 This short video is from Nigerian GPs working in the UK who have all had COVID-19 vaccines. Nigerian GP video.
This short video is from Nigerian GPs working in the UK who have all had COVID-19 vaccines. Nigerian GP video.
Please note this video is included as an information resource. It is not directly liked to HIV and i-Base was not involved in producing it.
Is there information about COVID-19 vaccines using sign language (BSL or ASL)?
Yes.
- SignHealth information about COVID-19 in BSL
- Direct link to SignHealth information focussed on vaccines
- US website in American Sign Language (ASL) with 22 videos on COVID-19
If the government didn’t protect me from the COVID-19 coronavirus, why should I trust them with the vaccine?
Perhaps luckily, the government are not directly involved in producing the vaccines. It is not directly running the studies that look at how well they work.
The government is also not directly involved in deciding which vaccines are approved. They have decided which ones are going to be used though – and we need to see if they have picked well.
Whether or not you use any medicine or vaccine is a decision that you make with your doctor as an individual.
I’ve experienced racism in the health system and receiving HIV care. How can you tell me this won’t be the same?
I am sorry for any previous experiences in your care. I am also sorry if you have not been treated fairly in the past.
Although I can not guarantee this will not happen again, there is a lot of information about how to deal with this.
I can however provide information on COVID-19 and the vaccines. This shows that the benefits of the vaccine so far are much greater than the risks from not getting the vaccine.
I hope that you reported previous incidents. There are support groups that can help make a complaint. You can also do this anonymously if you prefer.
Why did we get a COVID-19 vaccine so quickly, but there is still no vaccine for HIV?
There are several answers to this question – and many people are asking this.
Money and funding played a big part. More resources were focused on COVID-19. Whether rightly or wrongly, the urgency of COVID-19 led to a much larger budget. Luckily, this has been more effective than anyone first hoped.
There was rapid and close collaboration – between researchers, drug companies, health authorities and governments. Together of course with thousands of people who joined studies around the world.
COVID-19 is also and easier viral infection than HIV. SARS-CoV-2 does not hide inside the our DNA like HIV. The COVID-19 virus is also more stable. HIV changes very quickly to evade every vaccine so far invented.
But the question about HIV could be asked for every other infection where we are still waiting for a vaccine or for better treatment.
HIV does have at least 30 approved treatments. These mean we can now lead long and healthy lives.
The scientific advances made with COVID-19 will help other vaccines though, including HIV. For example, Moderna, the company behind one of the mRNA COVID-19 vaccines, plans to use the same advances for HIV.
Moderna press release from 11 January 2021.
As COVID vaccines are now becoming available, should I still join a study?
This is an important question because other vaccines are still being studied.
These include a vaccine from Janssen currently in phase 3 studies that should finish in January, and other studies that will continue for longer.
UCL is researching a self-amplifying ribonucleic acid (saRNA) vaccine.
Other research groups are looking at COVID-19 monoclonal antibodies instead of a vaccine.
Joining one of these studies might let you get vaccine or antibody protection before you are offered a COVID-19 vaccines by the NHS.
Talk though what this will involve if you are interested in a study. Some studies might limit how you use vaccines in the future. (See below).
Can I have a COVID-19 vaccine if I was already in a vaccine study?
Whether or not you can still benefit from the NHS vaccine programme will depend on the research study. It will also depend on whether you were in the active or control (placebo) group.
If the study tells you that you already received two doses of one of the currently approved vaccines, then you don’t need any further protection. This means that somebody else who still needs protection can use your NHS vaccine.
If you were in the control (placebo) group, then you can use the NHS vaccine. You might also have the chance to get the active vaccine used in the study.
If you only received one vaccine dose so far, then the study team can tell you now whether or not you got the active or placebo injection. You can then decide if you want the NHS vaccine now.
You might even produce important data from getting both types of vaccine. In practice, new studies will hopefully look at switching between different vaccines.
If the study was using monoclonal antibodies to COVID-19, and you received the active treatment, current advice is to wait at least 90 days before having a vaccine.
This early guidance is currently being reviewed so it is an important situation to discuss with your doctor.
If the vaccine is lifesaving, why is not available to everyone in the world?
You are right, for a vaccine to be really effective, everyone will need to use it. This includes in all countries.
Many organisations, including the World Health Organization (WHO), have been working all year to also make access fair.
For example, the international COVAX programme is aiming to vaccinate two billion people during 2021. This includes more than 100 low and middle income countries including across Africa, Asia and South America.
So optimistically, at some point, everyone will have access.
In practice, high income countries that could afford the first commercial vaccines have bought most of the first stock.
But some of the next stock during 2021 – and more importantly newer vaccines, will be available for the COVAX programme. This might not be until later in 2021 and 2022 though.
Where can I get more information?
The following links are to different sources for more information:
- i-Base run an information service if you have individual questions that you would like answered
- i-Base report news about COVID-19 treatment and vaccines in a monthly bulletin
- British HIV Association (for information about HIV and COVID-19)
- Q&A for doctors: similar Q with more technical answers
- UK patient information leaflet for the Pfizer/BioNTech vaccine (PDF)
- FDA 50-page document with detailed results on Pfizer vaccine
- YouTube website to watch the US CDC hearings for COVID vaccines
- A guide to vaccinology: from basic principles to new developments – comprehensive scientific review of safety and efficacy of vaccines published in Nature in December 2020
- Article on why vaccine is recommended for people with immune suppression and autoimmune conditions
- Guidance from the US on risk of allergic reactions to mRNA COVID-19 vaccines
- Website for WHO COVAX programme for global access
- The People’s Vaccine – a collaboration of large charities including Oxfam


Thanks for the response Simon. I feel far better equipped to respond to their claims now.
You’ve been a massive help, as always.
Hi Jason
Sorry for missing this Q – and actually whether people are doctors or not is not the point. i-Base is interested in accurate information, not who says it. Doctors are not necessarily right unless their info is up to date :)
The Pfizer/Moderna approach uses messenger RNA (mRNA), but none of this gets inside a person’s DNA. It contains instructions for the body to make proteins that look like coronavirus. These are what your immune system then responds to.
The part of the vaccine that carries these instructions then quickly gets broken down – a bit like a snapchat photo.
Nothing in the vaccine will get inside your DNA or change your DNA.
The amount of false information is difficult to understand after so many people have died from COVID-19 and the vaccines are amazingly safe and effective.
More than 100 million people globally have now been vaccinated and if there were problems they would have been reported immediately.
This is like a recipe for your body to make small proteins that look like the proteins on the surface of the coronavirus. This is very safe because nothing foreign goes into your body.
Hi Jason,
Are these people doctors?
Hi Simon, some people I work with are stating that the Pfizer/Moderna approach interferes with a person’s DNA.
What would be the best response to this?
What ingredients are used for developing this virus. Is DNA cloning part of this
Hi Mogamalie, the ingredients of each vaccine are listed in the patient leaflet that you should be given. Different vaccines use different technologies. The recently approved vaccines though are either mRNA (Pfizer and Moderna) or use an inactivated adenovirus to deliver a coronavirus protein (Oxford/AstraZeneca).