HIV pipeline report 2019
17 July 2019. Related: Special reports, Supplements, Pipeline report, Fit for purpose, Antiretrovirals.
 Simon Collins, HIV i-Base
Simon Collins, HIV i-Base
This is the third year that i-Base has produced the HIV pipeline review as part of our Fit for Purpose report on antiretroviral treatment optimisation.
Two versions are available:
- This full version includes more information on each drug, with full references.
- The “Pipeline-lite” version has a summary for each drug and is included in the i-Base Fit For Purpose report.
Both electronic versions (web and PDF) include hyperlinks to all research sources and references.
This review is based on HTB reports over the last year and coverage from CROI, IAS, EACS, Glasgow and other conferences. It also refers to some studies that will be presented at the AIDS 2019 conference being held in Mexico City from 21–24 July 2019.
Download HIV pipeline 2019 report (PDF) Download Pipeline-lite (PDF)
Introduction
Over the last year there were three new approvals of new drugs or fixed dose combinations (FDCs).
These included the new NNRTI doravirine (also in an FDC) and the dual FDC of dolutegravir/lamivudine. Also, although ibalizumab was approved in the US in March 2018 as the first monoclonal antibody, with an indication to treat multiple drug resistant HIV, approval in the EU is still pending as we went to press. (Table 1)
The FDA decision on the first injectable ART is expected by December 2019 with approval expected based on results from phase 3 studies. However, EU filing is only planned in Q32019 with a decision 12 months later.
And intriguing results from some broadly neutralising monoclonal antibodies (bNAbs) – notably those with long-acting formulations – show the potential to maintain viral suppression after ART has been stopped.
As with long-acting ARVs, these long-acting bNAbs also have important potential as PrEP.
The most quickly progressing compouns is islatravir (MK-8591), a newly-named NRTI with extremely high potency, in development both as treatment and PrEP and with a slow-release implant formulation that allows once-a-year administration.
Figure 1 updates the HIV pipeline by target and Tables 2 and 3 summarise compounds by development stage and likely use.
Finally, Table 4 highlights long-acting compounds. Over the next 5-10 years ART might become much simpler than taking a single pill once a day – with some compounds showing the potential for weekly, monthly, and perhaps even annual dosing.
Figure 1: HIV pipeline 2019: targets in the HIV lifecycle
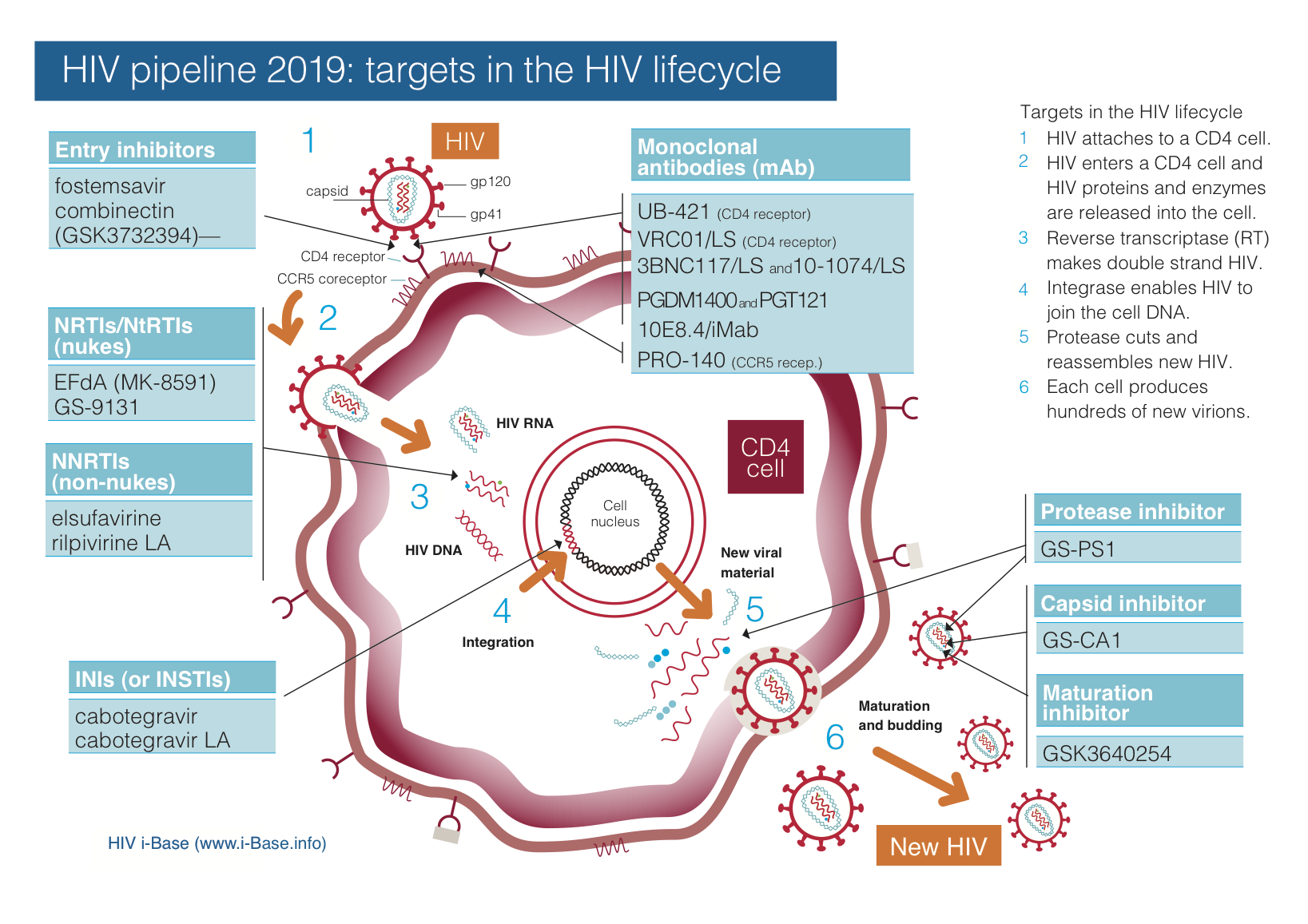
Key: INSTI: integrase strand transfer inhibitor; LA: long-acting; mAb: monoclonal antibody; NRTI: nucleoside/tide reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor.
Table 1: Recently approved and submitted HIV drugs
| Compound and company | Class | Comment |
| Approved in last year | ||
| doravirine and doravirine/TDF/3TCMerck/MSD |
NNRTI and NRTI FDC | Active against first generation NNRTI resistance. Non-inferior to efavirenz. FDC using doravirine with two generic NRTIs. Approved in US in August 2018 and EU in November 2018. |
| dolutegravir/3TC
ViiV Healthcare |
INSTI and NRTI FDC | Phase 3 GEMINI studies as initial ART are complete. Week-96 results expected at IAS 2019. Approved in the US in April 2019 and in the EU in July 2019. |
| ibalizumab (Trogarzo) Theratechnologies |
mAb | Approved by FDA in the US in March 2018 and given positive opinion in the EU in April 2019. Still awaiting EU final decision. Indication for multiple drug resistant HIV |
| Submitted applications or complete phase 3 | ||
| cabotegravir/rilpivirine long-acting (LA) injections ViiV Healthcare |
INSTI and NNRTI long acting FDC injections | Submitted to FDA in April 2019 with priority review expected December 2019. EU filing planned Q32019 with decision 12 months later. |
| fostemsavir (GSK3684934)
ViiV Healthcare |
ap120 attachment inhibitor | Phase 3 BRIGHTE study includes 96-week results (at IAS 2019) in treatment-experienced with extensive drug resistance. FDA submission planned Q4 2019 and EU in 2020. No information about priority review means likely 12 month decisions. |
Table 2: Likely positioning for new drugs
| Indication | Name |
| Treatment-naive | DTG/3TC; doravirine/3TC/TDF, MK-8591, GS-9131, ABX-464. |
| Switch option on ART | DTG/3TC; doravirine/3TC/TDF, MK-8591 etc. |
| Multidrug resistance (MDR) | ibalizumab, fostemsavir, MK-8591, GS-9131; ABX-464; all mAbs, likely other new compounds. |
| PrEP | CAB-LA; MK-8591; VRC01, other bNAbs, |
| Maintenance without ART | bNAbs – in combinations as swtich after viral load suppressed on ART. |
Recently approved new HIV drugs
Over the last year, three new drugs or FDCs were approved. See Table 1.
Although the PI-based FDC of darunavir/cobicistat/FTC/TAF (Symtuza) was approved in the US in July 2018, this had been approved in the EU six months earlier and was reported last year in the 2018 pipeline report.
Doravirine (Pifeltro) and doravirine/TDF/3TC (Delstrigo)
The NNRTI doravirine (Pifeltro) was also approved in the US (August 2018) and the EU (November 2018) together with an FDC (Delstrigo) combined with generic tenofovir DF (TDF) and generic lamivudine (3TC). [4, 5]
Doravirine is a once-daily NNRTI from Merck that can be taken with or without food. It has few drug interactions and retains activity against common first generation NNRTI mutations (K103N, Y181C, G190A and E138K).
Although current guidelines have moved to preferring integrase inhibitor-based first line treatment, NNRTIs are likely to still be used before protease inhibitors as alternatives.
Doravirine has a better tolerability profile compared to efavirenz (which is still widely-used despite the guidelines), but use might depend on being a less expensive option (including to integrase inhibitors).
Doravrine is being studied as part of a three-drug combination 3TC plus the investigational NRTI MK-8591. It is also being studied in dual combination with MK-8591.
Results from both these studies are due to be presented at IAS 2019 in two late-breaker presentations. [6, 7]
Dolutegravir/lamivudine (Dovato)
A dual combination of the integrase inhibitor dolutegravir with a single NRTI lamivudine was approved in the US in April 2019 and in Europe in July 2019. [8, 9]
This was based on phase 3 studies presented at AIDS 2018. [10]
The GEMINI studies showed that dual therapy with DTG/3TC was non-inferior to triple ART.
The disadvantages of dual therapy (for example, when HBV is a concern) are currently likely to outweigh advantages in low- and middle-income settings.
However, in high-income settings, with easier access to monitoring, the use for DTG/3TC is likely to be very different, especially if priced lower than other three-drug FDCs. The results are also encouraging for people who cannot use many other NRTIs.
A sub-study from the phase 3 ASPIRE study showed no differences in low levels viraemia <50 copies/mL in 2-drug vs 3-drug arms. [11]
GEMINI 96-week results are due to be presented at IAS 2019 together with another analysis of viral dynamics at low levels. [12, 13]
Ibalizumab (Trogarzo) – mAb – EU pending
Ibalizumab was approved by the US FDA in March 2018. Although given a positive opinion in the EU in April 2019, it is currently still awaiting a final decision in the EU. [14, 15]
Ibalizumab as the first monoclonal antibody to treat HIV positive people with multidrug resistance who are currently on failing ART.
Ibalizumab was developed by TaiMed Biologics. It is marketed in the US and Canada with the trade name Trogarzo by Theratechnologies. The US list price for ibalizumab is US $ 118,000 (WAC/Wholesale Acquisition Cost), which does not include costs for providing the infusions (the product is not self-administered). Easier to use formulations are also being studied.
Although this development took many years – with Phase 1b efficacy results first reported in 2008 – it is a considerable achievement for any compound to be the first drug approved in a new class.
Submitted applications or completed phase 3
Two new drugs and coformulations are already in late-stage development with regulatory applications submitted to the FDA and EU or phase 3 studies already completed.
Cabotegravir/rilpivirine long-acting (LA) injections
On 29 April 2019, ViiV Healthcare announced that the long-acting two-drug injection formulation of cabotegravir/rilpivirine had been submitted to the US FDA. [16]
The priority review means a decision is expected by the end of December 2019. EU filing was announced on 29 July 2019 with likely decision in 12 months.
Submission was based on 48-week results from the phase 3 FLAIR and ATLAS studies that were presented at CROI 2019. [17]
These studies reported >90% viral suppression to <50 copies/mL at week-48 meeting criteria for non-inferiority compared to three-drug oral therapy.
Cabotegravir (CAB) is a second-generation integrase inhibitor being developed as both an oral tablet and long-acting (CAB-LA) injectable formulation.
The oral formulation is primarily to use before using CAB-LA injections. CAB-LA is being studied both as treatment (coformulated with rilpivirine LA) and as single-drug for use as PrEP.
Both CAB formulations are being developed by ViiV Healthcare with the FDC in collaboration with Janssen.
CAB-LA has an extremely long half-life: a single injection resulted in drug levels that were still detectable in some people after more than a year. This requires an essential oral dosing lead-in phase before using the injection to screen for risk of a hypersensitivity reaction. The long half-life means that anyone stopping CAB-LA when used as treatment needs to switch to alternative ART (rather than interrupting treatment). When used as PrEP, current studies recommend switching to daily oral PrEP for a year.
However, a presentation at the HIVR4P conference in October 2018 reported cases where therapeutic levels of cabotegravir could still be detected after 2.5 years in men and 3.5 years in women. [18]
The oral formulation has a similar drug resistance profile to dolutegravir.
Pooled results from the FLAIR and ATLAS studies will be presented at IAS 2019, including presentations about participant quality of life using injections. [19, 20, 21]
Results will also be presented for a sustained release long acting cabotegravir implant to be used for PrEP. [22]
Fostemsavir – attachment inhibitor
Fostemsavir (GSK3684934) is an attachment inhibitor that binds to gp120 and prevents conformational changes needed for attachment.
It is active against nearly all HIV-1 subtypes, though not sub-type AE or group O and has no in vitro cross resistance to drugs from other classes.
This compound is being developed by ViiV after being acquired from BMS (BMS-663068).
Updated 48-week results were presented at Glasgow 2018 from the phase 3 BRIGHTE study. [23]
This was an advanced patient group with CD4 count at screening less than 200 cells/mm3 in 72% and 50 cells/mm3 in 41% of the group. Previous use of integrase inhibitors and protease inhibitors were reported for 80% and 96% respectively
At week 48, by snapshot analysis, 54% participants in the randomised study (146/272) and 38% (38/99) in the open label study had viral load <40 copies/mL. These were similar to rates at week 24.
Two posters were also presented at CROI 2019 supporting activity in treatment-experienced participants. [24, 25]
Currently, the submission is still being prepared for regulatory agencies, the timeline might be related to ViiV issues linked to scaling up manufacturing capacity. FDA submission planned Q4 2019 and EU in 2020. No information about priority review means likely 12 month decisions.
Two presentations at IAS 2019 will present 96-week results from the BRIGHTE study. [26, 27]
Compounds in phase 3 development
Two bNAbs are in phase 3 development although there have been little new data on both these compounds over the last year.
Leronlimab – mAb
Leronlimab (previously PRO 140) is a humanised IgG4 monoclonal antibody that blocks HIV entry by binding to CCR5 but is active against maraviroc-resistant virus.
Leronlimab has been in development for more than a decade, but that has been designated fast-track status, for potential to treat MDR HIV. In addition to use as an ARV in combination Leronlimab is also beings studied as a switch treatment after viral suppression on oral ART. Some people have reported sustained viral suppression for two years using weekly subcutaneous injections.
Preliminary results from a phase 2 study using this strategy were presented at CROI 2019. [28]
Unfortunately, this reported a high failure rate at the initial 350 mg dose that was not overcome with higher doses. Approximately 65% (149/226) of participants in the 350 mg, 33% (38/115) in the 525 mg arm and 14% (6/14) in the 700 mg arm, had confirmed viral rebound >200 copies/mL. Failure rates might increase further as some participants are still ongoing. Some of these participants are counted more than once as people with viral failure were given the option to return to ART or roll over to a higher dose of leronlimab. Resistance results from this use of monotherapy have not yet been presented.
Leronlimab is also being studied in non-HIV setting as prophylaxis against graft vs host disease (GVHD) in people undergoing allogenic stem cell transplant. [29]
Leronlimab is being developed by CytoDyn.
No further results are expected at IAS 2019.
UB-421 – mAb
UB-421 is a broadly neutralising mAb that targets CD4 binding with in vitro data that suggest comparable or greater potency compared to other compounds, including VRC01 and 3BNC117.
No new clinical data has been presented since 2017.
It is being developed by the Taiwanese company United BioPharma, with research sites in Taiwan. Although two phase 3 studies are listed to start in 2020, they were previously both due to start in 2018. Neither study is currently open to recruitment.
One is a randomised (1:2) open-label study in 375 participants on stable ART who will continue on current treatment or switch to monotherapy with UB-421. [30]
The second will add UB-421 or placebo to currently failing ART in 20 treatment experienced participants with drug resistance, followed by optimised background ART and open label UB-421 to all participants out to 435 weeks. [31]
The most recent data were presented at CROI 2017 but published in April 2019 in the NEJM. This was a phase 2 study in 29 virally suppressed participants on ART who used UB-421 monotherapy during an 8-week treatment interruption. UB-421 was given by infusion either 10 mg/kg weekly or 25 mg/kg every two-weeks. [32]
Although there were no cases of viral rebound during the monotherapy phase, viral load rebounded at 35 to 62 days after the last UB-421 dose in five participants who delayed restarting ART. All five later restarted ART and viral load became undetectable.
No further results are expected at IAS 2019.
Compounds in phase 1/2 studies
The compounds in this section include some that are likely to advance quickly into phase 3 studies and some where there has been little progress over the last year.
islatravir (MK-8591, EFdA) – NRTI
Islatravir (MK-8591) is a very interesting NRTI in development by Merck that is notable for high potency (currently using a 0.25 to 2.25 mg oral daily dose), a long plasma half-life that allows once-weekly oral dosing, a slow-release removable implant that might only require annual dosing and ongoing studies looking at use for both treatment and PrEP.
A single dose of islatravir (30 mg, 10 mg, 2 mg, 1 mg or 0.5 mg) in 30 treatment-naive participants (n=6 for each arm), produced mean viral load reductions at day 7 that were dose-related and ranged from approximately –1.2 logs (for the 0.5 mg, 1.0 mg and 2.0 mg groups) to approximately –1.6 logs (for the 10 mg and 30 mg group).
The latest results relating to potency and activity against drug resistance virus were presented in a poster at CROI 2019. [33]
This showed 4-fold lower IC50 for islatravir triphosphate than any other marketed NRTI with potential to use doses of 0.25 mg daily or 10 mg weekly. Common NRTI mutations, including M184I/V, K65R, and K70E, only confer low fold-shifts in antiviral potency and MK-8591 has greater inhibitory quotients against these drug-resistant mutations than those of TDF, TAF, and 3TC with WT HIV.
The potential for PrEP was shown using weekly oral doses of islatravir or placebo for three months in 16 macaques who were then exposed to rectal SIV (on day 6 of every weekly cycle) for 12 weeks, protecting all animals in the active arm. [34]
Preliminary results also suggested that a slow release implant might provide protection as PrEP for more than one year.
MK-8591 is also included in an FDC with 3TC and doravirine and is also being studied as dual therapy with doravirine. [35]
Three studies on islatravir will be presented at IAS 2019 including two late-breaker presentations on efficacy as treatment. [6, 7]
A third late-breaker abstract will present data on formulation in an annual implant for use as PrEP, extending dosing out to one year. [36]
GS-9131 – NRTI
GS-9131 is a prodrug of GS-9148 with early animal and in vitro drug resistance studies presented 12 years ago at CROI 2006. [37]
Other published studies highlight the potential for low risk of toxicity in animal studies and retains in vitro phenotypic sensitivity to broad NRTI resistance including mutations at K65R, L74V and M184V and multiple TAMS. [38]
The compound has good potency (EC50 = 25-200 nM) with activity against HIV-1 subtypes A, B, C, D, E, F, group O and N (EC50 0.29-113 nM), also against HIV-2. Synergistic activity was reported for GS-9131 in combination with AZT, FTC, abacavir, efavirenz, bictegravir, dolutegravir and lopinavir, and additive activity with TDF and TAF. [39]
Currently, the only ongoing study with GS-9131 is a phase 2 dose-finding trial Uganda in 58 treatment-experienced women who have detectable viral load >500 copies/mL on current NRTI-including ART. GS-9131 will be added as monotherapy (using 30 mg, 60 mg and 90 mg doses) for 10 days when background ART will be changed to bictegravir plus darunavir and ritonavir, with continued GS-9131. [40]
A poster presented at CROI 2019 reported on the high in-vitro threshold to drug resistance. [41]
No further results are expected at IAS 2019.
VRC01, VRC01LS and VRC07-523LS – bNAbs
VRC01 is a bNAb that targets the CD4 binding site that can be given by infusion or sub-cutaneous injection and that is in phase 1/2 development with multiple indications: for treatment, prevention and as a component of cure research.
Most ongoing studies are looking at VRC01 for HIV prevention, with two large international dose-finding, placebo-controlled phase 2 studies using VRC01 as PrEP are already ongoing that allow the option for participants to also use open-label oral TDF/FTC PrEP. [42, 43]
Although results are expected in 2019 there have always been concerns about using only a single mAb given the limited breadth and potency from one compound. Modelling suggests that nearly complete neutralisation of a given virus is needed for in vivo protection (~98% neutralisation for 50% relative protection) and that the inclusion of a second or third bNAb – is likely to be essential to provide cross-clade protection in African studies. [44]
A new long-acting formulation – VRC01LS – is also in phase 1 studies, designed to improve the half-life of the antibody, administered IV.
This includes using a single injection of VRC01LS in infants after birth to limit risk of vertical transmission and a potential role of additional injections for breastfed infants. [45]
Unfortunately, in a phase 1 study, VRC01 produced no additional impact on reducing the latently infected viral reservoir after being added to ART. VRC01 also had little impact on time to viral rebound after stopping ART, as part of a strategy in cure research.
A new long-acting formulation – VRC01LS – is also in phase 1 studies. [46, 47]
Results from the LS formulation will be presented at IAS 2019 together with VRC07-523LS – a variant long-acting bNAb. [48]
Other bNAbs: 3BNC117 and 10-1074; PGDM1400 and PGT121; 10E8
3BNC117 and 10-1074 are two broadly neutralising mAb’s that target CD4 binding that are in development at Rockefeller University.
Several phase 1 studies are using these individually and together and also in longer-acting versions that have an FcRn binding site mutation (LS) to improve pharmacokinetics. These are expected to extend the half-life by at least 4-fold, allowing monthly or two-monthly dosing.
An overview of latest results using this dual formulation was presented in one of the opening lectures to CROI 2019. [49]
This talk mainly looked at the potential for bNAbs to control HIV for extensive periods without ART, both in animal and human studies. [50, 51]
Another oral presentation at CROI 2019 included results from using a single subcutaneous injection of 10-1074 alone or in combination with 3BNC117 (10 mg each bNAb/kg) in a macaque study showed efficacy of these bNAbs as PrEP. [52]
3BNC117 is also included in a dual combination with the long-acting entry inhibitor albuvirtide that was approved last year in China. This phase 2 study with US study sites is looking at either 2-weekly or 4-weekly injection-based maintenance therapy. [53]
Also at CROI 2019, results from a phase 1 study using the bNAb PGT121 in treatment-naive participants, reported that a single infusion of PGT121 produced a median viral load reduction of –1.7 log copies/mL in participants with high baseline viral load, but breakthrough with bNAb resistance also occurred quickly when used as monotherapy. In two people starting with low baseline viral load (<400 copies/mL) a single infusion dropped viral load to undetectable where it remained, without ART, for at least eleven months. [54]
While the safety and tolerability of bNAbs are generally good, one study using the highly potent bNAb 10E8 was recently put on hold due to grade 3 skin erythema in 7/8 participants. Reactions occurred two days after receiving dual 10E8LS and VRC07 infusion (separately to each side of the stomach). These were associated with mild tenderness and fever (both transient) and confirmed by biopsy as panniculitis with lymphocytic inflammation (all cases resolved). This has been sufficient to put further clinical development of 10E8 on hold. [55]
The implications for the triple and trispecific studies that include 10E8 are unclear.
Preliminary results for a trispecific bNAb were presented at CROI 2019. This is the result of a joint development by the Vaccine Research Centre at NIAID and Sanofi where a single molecule could interact with three independent envelope regions: the CD4 binding site, MPER and the V1V2 glycan site. [56]
No further results are expected on these bNAbs at IAS 2019.
Elsulfavirine – NNRTI
Elsulfavirine (a prodrug of VM-1500A) is an NNRTI being developed by Viriom for registration in some middle-income countries.
Although limited data are available, in a randomised, double-blind phase 2b study conducted in Russia in 120 treatment naive participants, elsulfavirine 20 mg was compared to efavirenz 600 mg, each with TDF/FTC background NRTIs. The elsulfavirine arm reported similar viral suppression to <50 copies/mL (81% vs 73%), including those with baseline viral load >100,000 copies/mL (78% vs 62%), with fewer CNS side effects (32% vs 62%). [57]
A long-acting injectable formulation in development, with results from an animal study presented at IAS 2017, showing the potential for monthly by intramuscular (IM) or subcutaneous (SC) injection. [58]
Phase 2 results at 96-week were presented at AIDS 2018. [59] A second poster at IAS 2018 reported the potential for a long-acting injection formulation. [60]
However, no further results are expected at IAS 2019.
ABX464 – Rev inhibitor
ABX464 is an anti-inflammatory molecule thought to work by blocking the end stages of viral assembly. No new clinical data has been presented since 2017.
Although there are limited data as HIV treatment, results from a phase 2a dose-ranging study in 80 treatment-naive participants in Thailand reported 0.5 log copies/mL in 4/6 people at day 14 using the highest 150 mg dose as monotherapy (but with no response in 2/6). [61]
A phase 2b study looking at reducing the viral reservoir showed no change in time to viral rebound: 13 vs 14 days for days ABX464 vs placebo. [62]
An ongoing open-label phase 2 pharmacokinetic study in 36 HIV positive participants is currently ongoing, looking at 50 mg and 150 mg once-daily dosing. [63]
No further results are expected at IAS 2019.
GSK3640254 – maturation inhibitor
The maturation inhibitor GSK3640254 (previously BMS-986197) is currently in two phase 1 studies in HIV negative adults that include bioavailability of different formulations. [64, 65]
An earlier maturation inhibitor, BMS-955176, also acquired from BMS was discontinued in October 2016 due to gastrointestinal intolerability and treatment-emergent drug resistance.
Since the last pipeline report, results from an early phase 1 safety and telerability study were published last year. [66]
First results from a phase 2a study in HIV positive people were presented at CROI 2019. This included mean viral load reduction of –1.5 log copies/mL in the highest dose (200 mg/day) group. [67]
No further results are expected at IAS 2019.
Preclinical compounds of interest
As many companies do not widely publicise pre-clinical work, this section is restricted to a few studies. Apart from a few new compounds, this section is largely unchanged from the 2018 pipeline report.
Combinectin (GSK3732394) – adnectin/fusion inhibitor
Combinectin (GSK3732394, previously BMS-986197) is a combined adnectin/fusion inhibitor that stops viral entry by targeting multiple sites of action on gp41 and CD4.
This compound has the potential for self-administered once-weekly injections.
A summary of in vitro activity and resistance data and virologic data from mouse studies were presented at Glasgow 2016. [68]
In June 2019 the first phase 1 study in HIV negative volunteers started enrolling, with results expected mid-2020. [69]
No further results are expected at IAS 2019.
GS-PI1 – protease inhibitor
GS-PI1 is a once-daily unboosted protease inhibitor with high potency and a long half-life, and in vitro sensitivity against some second-generation PI resistance, in pre-clinical development by Gilead.
An oral presentation at CROI 2017 reported a high barrier to resistance both after in vitro passaging and against multiple resistance complexes from multiple PI-resistant clinical isolates, and pharmacokinetic data from rat and dog studies. [70]
However, no new data have been presented since and there are no listings on the clinical trials register for new studies.
No further results are expected at IAS 2019.
GS-CA1 – capsid inhibitor
First phase I data was presented at CROI 2019 on GS-CA1.
This is the first HIV capsid inhibitor, with a formulation that can be used for slow-release injections. [71]
Capsid is the cone-shaped structural core within the virion that protects HIV RNA and related enzymes. As part of a dynamic process, the capsid protein (p24) first breaks down to release viral contents into the CD4 cell to enable reverse transcription and also needs to reassemble inside new virions as part of the maturation process at the end of the lifecycle.
GS-CA1 acts in both the early and late stages by binding at a site that blocks both disassembly and assembly leading to defective new virions that are non-infectious.
The compound is potent with EC50 in target cells of 60 to 140 pM (compared to 1000 to 19000 for efavirenz, dolutegravir and atazanavir) with activity against drug resistance to current HIV classes. Although population sequencing showed the binding site to be highly conserved, capsid resistance can be generated from in vitro serial passaging.
The investigational compound is currently developed as a subcutaneous injection that in rat studies maintained plasma concentrations nine times above the protein adjusted EC95 ten weeks after a single injection. This suggests monthly or longer dosing intervals in humans.
A phase I study in HIV positive participants in currently ongoing with sites in the US. [72]
Early phase 1 results are expected as a late-breaker at IAS 2019.
MK-8583 (tenofovir prodrug), MK-8527 and MK-8558
Three compounds being developed by Merck are currently in phase I studies. Althouhg the first of these is an NRTI the trial listings do not include the mechanism of action. [73, 74, 75]
No further results are expected at IAS 2019 for any of these compounds.
Conclusion
The high number of recent approvals and ending applications for new HIV drugs is impressive. (see Table 1).
It is also important that this includes new classes that will overcome drug resistance to other classes and that additional new compounds are in development, especially those that are long-acting (see Tables 3 and 4).
This investment in formulations that use less than daily dosing could dramatically change the way that HIV is treated, with several of the compounds in this report already showing the potential for monthly or perhaps annual dosing.
Other companies are also looking to invest in similar technologies, reflecting that better HIV treatments is still seen as a competitative market. [76]
The global need for better HIV treatment also means that drugs developed in high-income countries need to have data to inform their use in all settings.
Table 3: HIV pipeline compounds by development phase
| Compound/ Company | Class | Notes |
| Phase 3 | ||
| cabotegravir
ViiV Healthcare |
INSTI | Oral formulation of integrase inhibitor mainly used for lead-in dose before long-acting formulation. Submitted to FDA in April 2019 and the EMA in July 2019. Also, long-acting implant for PrEP (phase1). |
| cabotegravir LA/ rilpivirine LA
ViiV Healthcare and Janssen |
INSTI | Injection with very long half-life – detectable after more than one year following single injection. Research as both treatment with rilpivirine LA and prevention as single compound. Submitted to FDA in April 2019 and the EMA in July 2019. |
| fostemsavir
ViiV Healthcare |
attachment inhibitor | Fostemsavir is a gp120 attachment inhibitor that is mainly being studied in treatment-experienced patients with MDR HIV in a large international study. Updated results at CROI 2019. Regulatory submission expected soon. |
| leronlimab CytoDyn | mAb CCR5 target | Once-weekly sub-cutaneous injection being studied in addition to ART for multi-drug resistance and as monotherapy maintenance therapy (without ART). Results at CROI 2019 showed high failure rate as monotherapy switch. |
| UB-421 United BioPharma | mAb CD4 binding | Infusion dosed either weekly or every two weeks as alternative to ART during treatment interruption. No recent results. |
| Phase 1/2 | ||
| islatravir (MK-8591, EFdA)
Merck/MSD |
NRTI | Highly potent, low dose, active against NRTI resistance. Long half-life, potential as oral (weekly dose) and implant (annual implant for PrEP). |
| MK-8591/3TC/doravirine
Merck/MSD |
FDC: NNRTI + 2 NRTIs | FDC with NNRTI doravirine and generic 3TC. Also as dual therapy with doravirine. Results presented at CROI 2019 and with two late-breakers at IAS 2019. |
| GS-9131
Gilead Sciences |
NRTI | Active against NRTI resistance. Synergy reported with AZT, FTC, abacavir, efavirenz, bictegravir, dolutegravir and lopinavir, and additive activity with TFV and TAF. Will be coformulated with other Gilead drugs. Phase 2 dose-finding study in Ugandan women. Potency data presented at CROI 2019. No results expected at IAS 2019. |
| VRC01 VRC01LS VRC07-523LS | bNAb CD4 binding | VRC01 ntravenous infusion (40 mg/kg) is being studied in cure research and as PrEP (2 large phase 3 studies AMP are ongoing). Also sub-cutaneous dosing of infants to prevent transmission at birth or from breastfeeding. VRC01LS is a long-acting formulation. Phase 1 results of VRC01LS and VRC07-523LS at IAS 2019. |
| 3BNC117 and 10-1074; PGDM1400 and PGT121, 10E8 etc. | bNAb | Many other bNAbs are in development, often in dual or triple combinations and including trispecific molecules. Potential to be used as switch option without ART and in current studies for use as PrEP. No results expected at IAS 2019. |
| elsulfavirine, prodrug of VM-1500A
Viriom |
NNRTI | NNRTI that is being developed for use in low and middle income countries. Similar activity to efavirenz. Long-acting formulation being studied with potential for monthly IM/SC injections. 96-week phase 2 results at AIDS 2018 together with potential for long-acting injectable formulation. No results expected at IAS 2019. |
| ABX464
Abivax |
Rev inhibitor | Compound with evidence of modest antiviral activity (~0.5 log in 4/6 people) that is also being studied for impact on the viral reservoir. Currently in phase 2. No new clinical data has been presented since 2017. No results expected at IAS 2019. |
| GSK3640254
ViiV Healthcare |
Maturation inhibitor | Maturation inhibitor with phase IIa results in HIV positive participants presented at CROI 2019: mean viral load reduction of –1.5 log copies/mL in the highest dose (200 mg/day) group. No results expected at IAS 2019. |
| Phase 1 and preclinical | ||
| combinectin (GSK3732394)
ViiV Healthcare |
Entry inhibitor gp41 & CD4 | Combined adnectin/fusion inhibitor that stops viral entry by targeting multiple sites of action and the potential for self-administered once-weekly injections. No results expected at IAS 2019. |
| GSPI1
Gilead Sciences |
Protease inhibitor | New QD unboosted PI, high potency, long half-life, potential in FDC single table regimen. No new clinical data has been presented since 2017. No results at IAS 2019. |
| GS-CA1
Gilead Sciences |
Capsid inhibitor | New class active at multiple stages of viral lifecycle. Sub-cutaneous injection with monthly or less frequent dosing. Phase I results in HIV negative at CROI 2019. Phase I in HIV positive participants is ongoing. |
| MK-8583, MK-8527, MK-8558 Merck/MSD | NRTI and others | These three compounds are registered for phase I studies in HIV positive particiapants, but with limited details on their mechanism of action. They are plausibly likely to have potential to be long-acting. No results expected at IAS 2019. |
Table 4: Compounds with long-acting formulations
| Compound | Company |
| cabotegravir/rilpivirine | ViiV/Janssen |
| cabotegravir implant for PrEP | ViiV |
| MK-8591 (EfDA) yearly implant for PrEP. Potentially weekly or longer dosing as treatment. | Merck/MSD |
| bNAbs: 3BNC117 and 10-1074; PGDM1400 and PGT121, 10E8 | Various including Rockefeller Institute. |
| combinectin | ViiV Healthcare |
| elsulfavirine | Viriom (Russia) |
| Gilead Sciences compounds – including CA1 (capsid inhibitor). | Gilead Sciences (in partnership with Lyndra) – compounds not specified [74] |
| MK-8583, MK-8527, MK-8558 | Merck/MSD. No details on compounds but likely to be long-acting. |
References
Key: CROI: Conference on Retroviruses and Opportunistic Infections; IAS: International AIDS Society; HIV Glasgow: Glasgow Congress on HIV Therapy.
- Huang Y. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016 Jun 16; 165(7): 1621–1631. doi: 10.1016/j.cell.2016.05.024.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4972332 - Ho D. First in human clinical evaluation of 10E8.4/iMab, a potent and broad bispecific antibody against HIV.
https://www.cavd.org/grantees/Pages/Grantee-Ho4.aspx - Wagh K et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathogens. March 5, 2018. DOI: 10.1371/journal.ppat.1006860.
http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1006860 - Merck (MSD) press release. European Commission approves Merck’s doravirine/lamivudine/tenofovir disoproxil fumarate (Delstrigo), a once-daily fixed-dose combination tablet as a complete regimen and doravirine (Pifeltro), an NNRTI, both for the treatment of HIV-1 in appropriate patients. (28 November 2018).
https://www.mrknewsroom.com - Merck (MSD) press release. FDA approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate), a once-daily fixed-dose combination tablet as a complete regimen and Pifeltrotm (doravirine), an NNRTI, both for the treatment of HIV-1 in appropriate patients. (30 August 2018).
https://www.mrknewsroom.com - Molina J-M et al. MK-8591 at doses of 0.25 to 2.25 mg QD, in combination with doravirine establishes and maintains viral suppression through 48 weeks in treatment-naïve adults with HIV-1 infection. IAS 2019. Late breaker abstract WEAB0402LB
http://programme.ias2019.org/Abstract/Abstract/4789 - Molina J-M et al. Tolerability, safety and efficacy of MK-8591 at doses of 0.25 to 2.25 mg QD, in combination with doravirine and lamivudine through 24 weeks in treatment-naïve adults with HIV-1 infection. IAS 2019. Late breaker abstract LBPED46.
http://programme.ias2019.org/Abstract/Abstract/4694 - FDA announcement listserve. FDA approves first two-drug complete regimen for HIV-infected patients who have never received antiretroviral treatment. (8 April 2019).
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm635526.htm - ViiV press statement. ViiV Healthcare receives EU Marketing Authorisation for Dovato (dolutegravir/lamivudine), a new once-daily, single-pill, two-drug regimen for the treatment of HIV-1 infection. (03 July 2019).
https://www.gsk.com/en-gb/media/press-releases/viiv-healthcare-receives-eu-marketing-authorisation-for-dovato-dolutegravirlamivudine-a-new-once-daily-single-pill-two-drug-regimen-for-the-treatment-of-hiv-1-infection - Cahn P et al. Non-inferior efficacy of dolutegravir (DTG) plus lamivudine (3TC) versus DTG plus tenofovir/emtricitabine (TDF/FTC) fixed-dose combination in antiretroviral treatment-naïve adults with HIV-1 infection – 48-week results from the GEMINI studies. AIDS 2018, 23-27 July 2018, Amsterdam. Late breaker oral abstract TUAB0106LB.
http://programme.aids2018.org/Abstract/Abstract/13210 (abstract)
https://youtu.be/pgmb1Fi63Fo?t=3642 (webcast) - Taiwo B et al. No significant changes to residual viremia after switch to dolutegravir and lamivudine in a randomized trial. Glasgow HIV Congress 2018, 28 – 31 October 2018. Oral abstract O145.
- Cahn P et al. Durable efficacy of dolutegravir (DTG) plus lamivudine (3TC) in antiretroviral treatment-naïve adults with HIV-1 infection – 96-week results from the GEMINI studies. IAS 2019. Late breaker abstract WEAB0404LB
http://programme.ias2019.org/Abstract/Abstract/4767 - Underwood P et al. Dolutegravir (DTG) plus lamivudine (3TC) versus DTG plus tenofovir/emtricitabine (TDF/FTC) fixed-dose combination in the GEMINI studies – viral load rebound including ‘blips’ through 48 weeks. IAS 2019. Poster abstract MOPEB231.
http://programme.ias2019.org/Abstract/Abstract/3645 - FDA press release. FDA approves new HIV treatment for patients who have limited treatment options. (06 March 2018).
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm599657.htm? - Theratechnologies press statement. Trogarzo receives positive recommendation from scientific advisory group of CHMP in Europe. (11 April 2018)
https://theratechnologies.s3.amazonaws.com/prod/media/SAG_Decision_April_11_E.pdf - ViiV press statement. ViiV Healthcare submits New Drug Application to US FDA for the first monthly, injectable, two-drug regimen of cabotegravir and rilpivirine. (29 April 2019).
https://www.viivhealthcare.com/en-gb/media (direct link) - Collins S. Phase 3 results with dual therapy cabotegravir/rilpivirine long-acting injections: ATLAS and FLAIR studies. HTB: 20(4), 12 March 2019.
https://i-base.info/htb/35812 - Collins S. Cabotegravir levels can be detected several years: PK tail to be covered by oral PrEP. HTB 19(17) 13 November 2018.
https://i-base.info/htb/35212 - Overton ET et al. Monthly long-acting cabotegravir and rilpivirine is non-inferior to oral ART as maintenance therapy for HIV-1 infection: Week 48 pooled analysis from the Phase 3 ATLAS and FLAIR studies. IAS 2019. Poster abstract MOPEB257.
http://programme.ias2019.org/Abstract/Abstract/1291 - Murray M et al. Patient views on long acting HIV treatment: Cabotegravir + rilpivirine as maintenance therapy (ATLAS 48 week results). IAS 2019. Oral abstract MOAB0103.
http://programme.ias2019.org/Abstract/Abstract/3680 - Murray M et al. Patient reported outcomes on long-acting Cabotegravir + Rilpivirine as maintenance therapy: FLAIR 48 week results. IAS 2019, Poster abstract MOPEB258.
http://programme.ias2019.org/Abstract/Abstract/2373 - Pons-Faudoa F et al. Sustained release of cabotegravir from nanochannel delivery implant for HIV PrEP. IAS 2019. Poster abstract TUPEA106.
http://programme.ias2019.org/Abstract/Abstract/2369 - Aberg J et al. Week 48 safety and efficacy of the HIV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced participants (BRIGHTE study). Glasgow HIV Congress 2018, 28 – 31 October 2018. Oral abstract O344A.
- Thompson M et al. Long-term safety & efficacy of fostemsavir in treatment-experienced HIV participants. CROI 2019. Poster abstract 483. http://www.croiconference.org/sessions/long-term-safety-efficacy-fostemsavir-treatment-experienced-hiv-participants
- Saladini F et al. Genotypic and phenotypic susceptibility to fostemsavir in multidrug-resistant HIV-1. CROI 2019. Poster abstract 548. http://www.croiconference.org/sessions/genotypic-and-phenotypic-susceptibility-fostemsavir-multidrug-resistant-hiv-1
- Lataillade M et al. Week 96 safety and efficacy of the novel HIV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced participants infected with multi-drug resistant HIV-1 (BRIGHTE study). IAS 2019. Oral abstract MOAB0102.
http://programme.ias2019.org/Abstract/Abstract/3372 - Ackerman P et al. A subgroup analysis of the week 96 efficacy and safety results evaluating fostemsavir in heavily treatment-experienced HIV-1 infected participants in the phase 3 BRIGHTE study: Results from the randomized cohort. IAS 2019. Poster abstract MOPEB234.
http://programme.ias2019.org/Abstract/Abstract/4169 - Dhody K et al. PRO 140 SC: Long-acting, single-agent maintenance therapy for HIV-1 infection. CROI 2019. Poster abstract 486.
http://www.croiconference.org/sessions/pro-140-sc-long-acting-single-agent-maintenance-therapy-hiv-1-infection - ClinicalTrials.gov. Study of PRO 140 for prophylaxis of acute GVHD in patients with AML or MDS undergoing allogeneic stem-cell transplant. (GVHD). NCT02737306.
https://clinicaltrials.gov./ct2/show/NCT02737306 - ClinicalTrials.gov. To investigate the efficacy and safety of UB-421 monotherapy in HIV-1 infected adults. NCT0314921.
https://clinicaltrials.gov/ct2/show/NCT0314921 - ClinicalTrials.gov. UB-421 combine with optimized background therapy regimen in multi-drug resistant HIV-1 infection patients. NCT03164447.
https://clinicaltrials.gov/ct2/show/NCT03164447 - Wang C-Y et al. Effect of anti-CD4 antibody UB-421 on HIV-1 rebound after treatment interruption. N Engl J Med 2019; 380:1535-1545. DOI: 10.1056/NEJMoa1802264
https://www.nejm.org/doi/full/10.1056/NEJMoa1802264?url_ver=Z39.88-2003 - Grobler J et al. MK-8591 potency and PK provide high inhibitory quotients at low doses QD and QW. CROI 2019. Poster abstract 481.
http://www.croiconference.org/sessions/mk-8591-potency-and-pk-provide-high-inhibitory-quotients-low-doses-qd-and-qw - Markowitz M et al. Low dose MK-8591 protects rhesus macaques against rectal SHIV infection. CROI 2018, Boston. Oral abstract 89LB.
www.croiconference.org/sessions/low-dose-mk-8591-protects-rhesus-macaques-against-rectal-shiv-infection (abstract)
www.croiwebcasts.org/console/player/37191 (webcast) - ClinicalTrials.gov. MK-8591 with doravirine and lamivudine in participants infected with HIV type 1 (MK-8591-011) (DRIVE2Simplify). NCT03272347.
https://clinicaltrials.gov/ct2/show/NCT03272347 - Matthews RP et al. First-in-human trial of MK-8591-eluting implants demonstrates concentrations suitable for HIV prophylaxis for at least one year. IAS 2019. Late breaker abstract TUAC0401LB.
http://programme.ias2019.org/Abstract/Abstract/4843 - Cihlar T et al. GS9148: A novel nucleotide active against HIV-1 variants with drug-resistance mutations in reverse transcriptase. 13th Conference on Retroviruses and Opportunistic Infections, 5-8 February 2006, Denver. Oral abstract 45. (Abstract no longer online).
- Cihlar T et al. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of HIV Type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob Agents Chemother. 2008 Feb; 52(2): 655–665. Published online 2007 Dec 3. doi: 10.1128/AAC.01215-07.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2224772 - White KL et al. GS-9131 is a novel NRTI with activity against NRTI-resistant HIV-1. CROI 2017, 13-16 February, Seattle. Poster abstract 436.
http://www.croiconference.org/sessions/gs-9131-novel-nrti-activity-against-nrti-resistant-hiv-1 - ClinicalTrials.gov. Efficacy of GS-9131 functional monotherapy in HIV-1-positive adults failing a nucleos(t)Ide reverse transcriptase inhibitor-containing regimen. NCT03472326.
https://clinicaltrials.gov/ct2/show/NCT03472326 - Ibanescu R-I et al. Favourable outcome of in vitro selections with novel NRTI prodrug GS-9131. CROI 2019. Poster abstract 482.
http://www.croiconference.org/sessions/favourable-outcome-vitro-selections-novel-nrti-prodrug-gs-9131 - ClinicalTrials.gov. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection in women. NCT02568215.
https://www.clinicaltrials.gov/ct2/show/NCT02568215 - ClinicalTrials.gov. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection among men and transgender persons who have sex with men. NCT02716675.
https://www.clinicaltrials.gov/ct2/show/NCT02716675 - Wagh K et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathogens. March 5, 2018. DOI: 10.1371/journal.ppat.1006860.
http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1006860 - McFarland E et al. Safety and pharmacokinetics of monoclonal antibody, VRC01LS, in HIV-exposed newborns. CROI 2019. Oral abstract 45.
http://www.croiconference.org/sessions/safety-and-pharmacokinetics-monoclonal-antibody-vrc01ls-hiv-exposed-newborns - ClinicalTrials.gov. Evaluating the safety and pharmacokinetics of VRC01 and VRC01LS, potent anti-HIV neutralizing monoclonal antibodies, in HIV-1-exposed infants. NCT02256631.
https://clinicaltrials.gov/ct2/show/NCT02256631 - ClinicalTrials.gov. Safety and virologic effect of a human monoclonal antibody, VRC-HIVMAB080-00-AB (VRC01LS), with broad HIV-1 neutralizing activity, administered intravenously to HIV-positive adults. NCT02840474.
https://clinicaltrials.gov/ct2/show/NCT02840474 - Chen G et al. Safety and virologic effect of the HIV-1 broadly neutralizing antibodies, VRC01LS or VRC07-523LS, administered to HIV-infected adults in a phase 1 clinical trial. IAS 2019. Late breaker abstract WEAA0305LB.
http://programme.ias2019.org/Abstract/Abstract/4941 - Nussensweig M et al. Discovery and development of HIV broadly neutralizing antibodies. Opening session. CROI 2019, 4-7 March 2019, Seattle. Oral abstract 10.
http://www.croiwebcasts.org/console/player/41037 (webcast) - Nishimura Y et al. Early antibody therapy can induce long lasting immunity to SHIV. Nature 2017; 543(7646): 559–563. doi: 10.1038/nature21435
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5458531 - Mendoza et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature (561):479–484 (2018).
https://www.nature.com/articles/s41586-018-0531-2 - Garber DA et al. Protection against penile or intravenous SHIV challenges by bNAb 10-1074 or 3BNC117. CROI 2019, 4-7 March 2019, Seattle. Oral abstract 100.
http://www.croiconference.org/sessions/protection-against-penile-or-intravenous-shiv-challenges-bnab-10-1074-or-3bnc117 (abstract)
http://www.croiwebcasts.org/p/2019croi/100 (webcast) - ClinicalTrials.gov. Albuvirtide and 3BNC117 as long-acting maintenance therapy in virologically suppressed subjects (ABL). NCT03719664.
https://clinicaltrials.gov/ct2/show/NCT03719664 - Stephenson KE et al. Therapeutic activity of PGT121 monoclonal antibody in HIV-infected adults. CROI 2019, 4-7 March 2019, Seattle. Oral abstract 145.
http://www.croiconference.org/sessions/therapeutic-activity-pgt121-monoclonal-antibody-hiv-infected-adults (abstract)
http://www.croiwebcasts.org/p/2019croi/145 (webcast - Koup R. Review of bNAbs in clinical development. R4P2018, 21-25 October 2018. HVTN and HPTN satellite session. 21 October, 4.00 pm.
http://webcasts.hivr4p.org/console/player/40515 (webcast) - Pegu A et al. Potent antiviral activity of trispecific broadly neutralizing HIV antibodies. CROI 2019, Seattle. Late breaker oral abstract 28 LB.
http://www.croiconference.org/sessions/potent-antiviral-activity-trispecific-broadly-neutralizing-hiv-antibodies (abstract)
http://www.croiwebcasts.org/p/2019croi/28 (webcast) - Murphy R et al. Elsulfavirine as compared to efavirenz in combination with TDF/FTC: 48-week study. CROI 2017, 13-16 February, Seattle. Late breaker abstract 452LB.
http://www.croiconference.org/sessions/elsulfavirine-compared-efavirenz-combination-tdfftc-48-week-study - Bichko V et al. Pre-clinical pharmacokinetics of elsulfavirine/VM1500A long acting injectable formulations. IAS 2017, 23-26 July, Paris. Poster abstract WEPEA0190.
http://programme.ias2017.org/Abstract/Abstract/1515 - Murphy R et al. Elsulfavirine-based antiretroviral treatment in combination with two NRTIs: 96 weeks. IAS 2018. Poster abstract THPEB043.
http://programme.aids2018.org/Abstract/Abstract/7018 - Koryakova A et al. Pharmacokinetics of VM1500A long acting injectable formulations for HIV-1 infections treatment and prevention after repeat-dose administration in dogs. IAS 2018. Poster abstract THPEA013
http://programme.aids2018.org/Abstract/Abstract/12143 - Scherrer D et al. Early evidence of antiviral activity & safety of ABX464 in HIV treatment-naïve patients. CROI 2016, February 22–25, Boston, Late breaker poster abstract 461LB.
http://www.croiconference.org/sites/default/files/posters-2016/461LB.pdf (PDF) - Paredes R et al. ABX464 decreases total HIV DNA in PBMC´s when administered during 28 days to HIV-infected patients who are virologically suppressed. IAS 2017, 23-26 July, Paris. Poster abstract TULBPEB22.
http://programme.ias2017.org/Abstract/Abstract/5650 - ClinicalTrials.gov. An open-label study of the safety, pharmacokinetics, and pharmacodynamics of ABX464 in seronegative and seropositive adults. NCT02990325.
https://clinicaltrials.gov/ct2/show/NCT02990325 - ClinicalTrials.gov. GSK3640254 first time in human (FTIH) study in healthy volunteers. NCT03231943.
https://clinicaltrials.gov/ct2/show/NCT03231943 - ClinicalTrials.gov. A study to compare the relative bioavailability of two different formulations of GSK3640254. NCT03575962.
https://clinicaltrials.gov/ct2/show/NCT03575962 - Johnson M et al. The safety, tolerability, and pharmacokinetic profile of GSK2838232, a novel 2nd generation HIV maturation inhibitor, as assessed in healthy subjects. Pharmacol Res Perspect. 2018 Jul; 6(4): e00408. doi: 10.1002/prp2.408
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5989765 - DeJesus E et al. A phase IIa study of novel maturation inhibitor GSK2838232 in HIV patients. CROI 2019, Seattle. Oral abstract 142.
http://www.croiconference.org/sessions/phase-iia-study-novel-maturation-inhibitor-gsk2838232-hiv-patients (abstract)
http://www.croiwebcasts.org/console/player/41311 (webcast) - Krystal M et al. HIV combinectin GSK3732394: a long-acting inhibitor with multiple modes of action. Glasgow 2016.
http://www.natap.org/2016/GLASGOW/GLASGOW_27.htm - ClinicalTrials.gov. Evaluation of the safety, tolerability and pharmacokinetics (PK) of GSK3732394 first-time-in-human (FTIH) study
https://clinicaltrials.gov/ct2/show/NCT03984812 - Link JO et al. Novel HIV PI with high resistance barrier and potential for unboosted QD oral dosing. CROI 2017, 13-16 February, Seattle. Late breaker abstract 433.
http://www.croiwebcasts.org/p/2017croi/croi33636 - Sage JE et al. Safety and PK of subcutaneous GS-6207, a novel HIV-1 capsid inhibitor. CROI 2019, oral abstract 141.
http://www.croiconference.org/sessions/safety-and-pk-subcutaneous-gs-6207-novel-hiv-1-capsid-inhibitor - ClinicalTrials.gov. Safety, pharmacokinetics, and antiviral activity of GS-6207 administered subcutaneously in HIV-1 infected adults.
https://clinicaltrials.gov/ct2/show/NCT03739866 - ClinicalTrials.gov. MK-8583 single dose study in HIV-1 infected participants (MK-8583-002).
https://clinicaltrials.gov/ct2/show/NCT03552536 - ClinicalTrials.gov. A Study of MK-8527 in HIV-1 infected participants (MK-8527-002).
https://clinicaltrials.gov/ct2/show/NCT03615183 - ClinicalTrials.gov. Safety, tolerability, pharmacokinetics, and anti-retroviral activity of MK-8558 monotherapy in anti-retroviral-naïve HIV-1 infected participants (MK-8558-002).
https://www.clinicaltrials.gov/ct2/show/NCT03859739 - Lyndra press statement. Lyndra Therapeutics and Gilead Sciences to collaborate on development of ultra-long-acting HIV therapeutics. (09 July 2019).
https://lyndra.com/news/lyndra-therapeutics-and-gilead-sciences-to-collaborate-on-development-of-ultra-long-acting-hiv-therapeutics

