Preventive technologies, immune-based and gene therapies, and research towards a cure
14 July 2011. Related: HIV prevention and transmission, Basic science and immunology, Vaccines and microbicides.
Richard Jefferys
The phrase “product pipeline” typically conjures up the notion of multiple experimental candidates incrementally advancing along a pre-plumbed path toward licensure and widespread availability. But for most of the approaches described in this section of the report, the route toward a pharmacy shelf is far more convoluted and uncertain. Few large pharmaceutical companies are involved in the development of the candidates listed here; the majority are collaborative efforts between small biotech firms, academic researchers, non-profits, and government funders. And even those with the support of a major manufacturer can face unique obstacles related to their novelty, because there are as yet no approved precedents in any of these realms.
The current state of the biomedical prevention pipeline offers illustrative examples. After decades of disappointment and frustration, the past few years have seen low but statistically significant efficacy reported for each of the main approaches: vaccines, microbicides and, most recently, pre-exposure prophylaxis (PrEP). The vaccine trial, named RV144, involved an ALVAC canarypox vector made by Sanofi-Pasteur combined with an envelope protein booster shot, AIDSVAX. While Sanofi-Pasteur remains committed to following up on the marginal degree of protection (31%) observed among recipients of the regimen (Rerks-Ngarm 2009), the company that made AIDSVAX, VaxGen, ceased to exist several years ago after the product failed to show efficacy given alone. Attempts to duplicate and improve upon the results have thus been slowed by the need to secure a new manufacturer for the envelope protein boost.
Greater success was reported last year with a microbicide consisting of a gel form of the antiretroviral drug tenofovir (Viread), which demonstrated 39% protective efficacy in the CAPRISA 004 trial in South Africa (Abdool Karim 2010). However, the next steps toward licensure have proven surprisingly slippery. The US Food and Drug Administration (FDA) has indicated that at least one more confirmatory trial (in addition to an ongoing study called VOICE) will be sufficient for them to consider the product for approval, but securing the relatively small amount of funding necessary for the new efficacy evaluation proved difficult and time-consuming. The trial, FACTS 001, is now expected to get underway in August 2011. The maker of Viread, Gilead Sciences, has licensed the gel form to the non-profit organization CONRAD, so the development of the microbicide also represents a test case for the viability of non-profit manufacturing and marketing.
Among the most significant biomedical prevention news since the last TAG pipeline report in 2010 was the announcement of the long-awaited first efficacy results of PrEP in HIV negative gay men and transsexual women at high risk of infection (Grant 2010). The iPrEx study found that individuals assigned to receive Truvada (a pill combining two antiretroviral drugs, tenofovir and emtricitabine) experienced a 44% reduction in risk of HIV infection, with additional analyses indicating that protection was significantly better among participants who closely adhered to the regimen. While Gilead donated Truvada for this and other PrEP studies, it was not otherwise involved and it was unclear whether the company would pursue a prevention indication for the drug. After the iPrEx findings were announced, Gilead expressed its intent to submit the data to FDA for consideration, which provoked a vociferous and at times acrimonious debate regarding whether such a filing would be appropriate or premature. Subsequently, the picture was further complicated when news emerged that a trial of Truvada as PrEP in women was being stopped after an interim analysis found it would be unable to show efficacy. A broad lesson from all these biomedical prevention developments is that an approach can get tantalising close to the end of the pipeline, yet still face significant impediments to actually emerging from it.
Immune-based therapies (IBTs) and gene therapies for HIV have long been entrenched in a distant corner of the research field. This is partly due to uncertainties about mechanisms of action and how best to define and measure success, particularly in light of the dramatically beneficial effects of HIV suppression with antiretroviral drugs. But resurgent interest in curing HIV infection is now helping to move these types of approaches toward the mainstream. In particular, the widely reported case of Timothy Brown, who has remained off antiretroviral therapy and free of detectable HIV for four years and counting after a complex series of high-risk treatments for cancer – including stem cell transplants from a donor lacking the CCR5 receptor – is viewed as a compelling proof of concept that a cure for chronic HIV infection is possible (Allers 2011). The goals for potentially curative therapies are relatively straightforward: either eradicate HIV completely (to the extent that this can be verified with current testing technologies) or induce long-term control of the virus in the absence of ongoing treatment (referred to as a functional cure). In addition to IBTs and gene therapies, cure research includes treatments – most notably histone deacetylase (HDAC) inhibitors – that aim to awaken the latent HIV that otherwise can persist for life in dormant form, integrated into the host cell’s DNA, invisible to the immune system, yet subject to reactivation by immune stimuli or to renewed replication when the resting infected cell divides.
Another potential role for IBTs and gene therapies is addressing the immune system dysfunction that can persist in some individuals despite HIV suppression. Examples include inadequate CD4 T cell recovery, elevated levels of immune activation and inflammation, and an accelerated aging of the immune system called immunosenescence. Studies have linked all of these phenomena to an increased risk of ill health (Marin 2009; Tan 2008; Tien 2010; Deeks 2011), suggesting that an IBT and/or gene therapy capable of addressing them could conceivably offer clinical benefits.
Table 1. HIV vaccines pipeline 2011
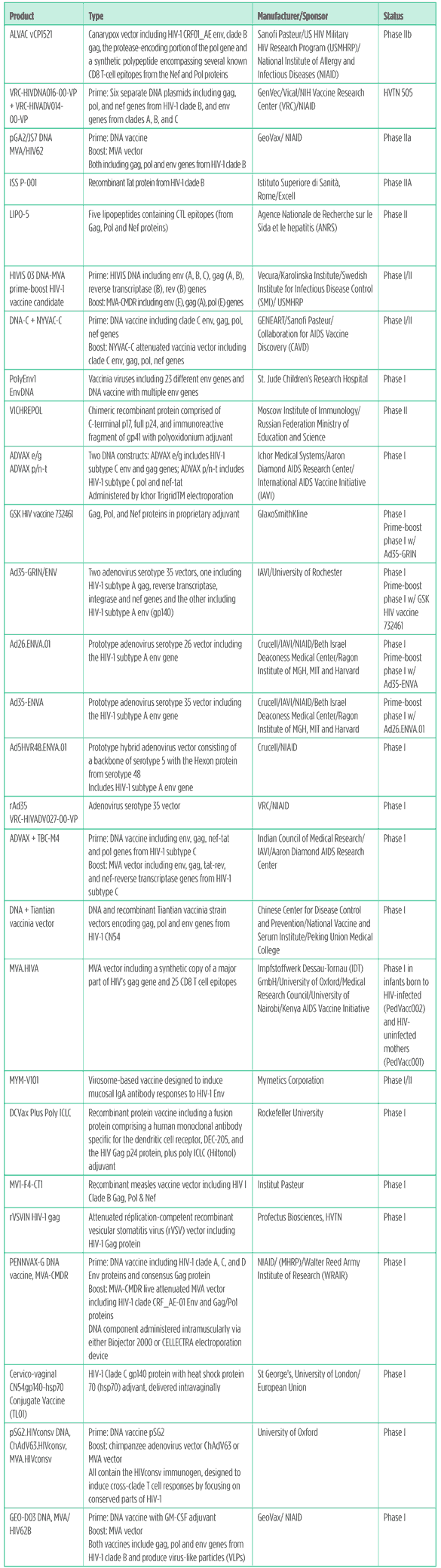
Spurred by the borderline but statistically significant protection observed in the RV144 trial of ALVAC/AIDSVAX, HIV vaccine research continues to move ahead on multiple fronts.
Identifying correlates of protection in RV144
Many scientists are engaged in the search for immunological markers that might have been linked to protection against HIV in the RV144 trial. Identification of such ?correlates of protection? is one of the Holy Grails of vaccine research and currently, according to Jerome Kim from the US HIV Military HIV Research Programme, 35 investigators from 20 institutions are working on 32 different assays that could potentially be used to analyze RV144 samples (Kim 2011). Data from this work should start to become available toward the end of 2011. In the meantime, Kim and colleagues have unveiled some of their results hinting that CD4 T cells targeting the V2 region of the HIV envelope could have played a role in the trial outcome (Currier 2011).
Replicating and extending the RV144 results
While the vaccine field has been buoyed by RV144, there remains a deflating possibility that the observed evidence of protection was not a consequence of immunization, but simply a result of chance. A recently published statistical reevaluation of the efficacy result argues there is a 22% or greater probability it was spurious, which the authors note is ?an inference that reflects greater uncertainty than has much of the discussion about this trial? (Gilbert 2011).This uncertainty emphasises the importance of efforts to try and replicate and extend the RV144 findings.
The HIV Vaccine Trials Network (HVTN) has published plans for adaptive trial designs (Corey 2011) which will be used to rapidly evaluate a variety of prime-boost vaccine regimens in the high prevalence setting of South Africa. The US HIV Military Research Programme is also planning a new efficacy trial in men who have sex with men (MSM) in Thailand, using the same or a similar regimen to RV144 but with an additional booster immunization at the 12 month time point (the last shot in RV144 was at six months). This trial is slated to begin in 2014 (Kim 2011).
There is only one ongoing HIV vaccine efficacy trial, HVTN 505. It involves a prime-boost regimen comprising a DNA vaccine followed by an adenovirus serotype 5 (Ad5) vector.The target population is circumcised MSM and male-to-female (MTF) transgender persons who have sex with men. The design of the trial has gone through myriad iterations, and until recently the primary goal was to look at whether the vaccines reduced viral load in recipients who subsequently acquired HIV. In light of the RV144 results, consideration is now being given to expanding HVTN 505 in size so that the effect of vaccination on risk of HIV acquisition can also be evaluated.
Developing new vectors, immunogens and adjuvants
As the term implies, vectors are delivery vehicles – often weakened forms of viruses – that carry vaccine ingredients into the body. Immunogens are the ingredients derived from HIV that the vaccine aims to induce immune responses against, and adjuvants are substances designed to enhance the magnitude and/or quality of those immune responses. The HIV vaccine pipeline contains a variety of vector/immunogen/adjuvant combinations, most commonly administered in prime-boost regimens. New vectors in human trials in 2011 include measles virus, vesicular stomatitis virus (VSV), and a chimpanzee adenovirus (Lorin 2004; Cooper 2008; Rosario 2010). Also in the mix are vaccines that deliver proteins or protein fragments directly, similar to the AIDSVAX envelope protein vaccine used as a booster shot in the RV144 trial.
A novel HIV vaccine vector that has received widespread media coverage due to promising results in macaques is cytomegalovirus (CMV). The vector is under development by the Vaccine and Gene Therapy Institute (VGTI) in collaboration with the International AIDS Vaccine Initiative (IAVI), but has not yet entered human testing. In a study published in the journal Nature, the use of CMV as an SIV vaccine vector led to an unprecedented degree of immunological control of a highly pathogenic challenge virus, SIVmac251 (Hansen 2011). Although large swathes of the human population are already infected with CMV, pre-existing immunity to the vector is not considered an issue because the virus has evolved immune evasion mechanisms that allow it to re-infect (Hansen 2010). There is, however, an important caveat about the use of CMV that was conspicuously absent from press reports about this study; over the last couple of decades, evidence has accumulated that CMV infection has an array of pernicious long-term effects on human health, contributing to cardiovascular disease (Stassen 2006), earlier mortality (Simanek 2011), and a type of immune system damage called immunosenescence that is associated with morbidity and mortality as people reach old age (Pawelec 2011). Although researchers are attempting to render CMV vectors safe for human use, it is currently unclear if – and how – safety can be sufficiently demonstrated to allow clinical trials.
New approaches to immunogen design attempt to improve the ability of vaccines to induce immune responses against a broad array of HIV targets. Oxford University and Tomas Hanke are testing HIVconsv, an immunogen incorporating fourteen parts of HIV that are highly conserved among multiple different clades (L?urneau 2007). Mosaic HIV immunogens represent another approach with the same goal; human testing is anticipated to start within the next year (Corey 2010).
Adjuvants that have ambled into clinical trials since the last TAG pipeline report include heat shock protein 70 (Hsp70), a naturally occurring protein under study as an enhancer of mucosal immune responses (Lehner 2004), and the cumbersomely-named cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF for short), which the company GeoVax is investigating as an adjuvant for its DNA/MVA vaccine after obtaining promising results in macaques (Lai 2011).
The multitude of candidates in the HIV vaccine pipeline prompts the question of how products will be selected for advancement into efficacy trials. At one time, the major criteria were the nature and magnitude of the anti-HIV immune responses invoked by the vaccines in early studies, along with evidence from pre-clinical research in the SIV/macaque model. However, one of the implications of the RV144 trial is that current immune response assays and animal models may not necessarily predict protective efficacy in humans (the ALVAC/ AIDSVAX combination performed dismally by both criteria). It has also become clear that ostensibly similar regimens can induce immune responses that differ substantially in quality, with unclear implications for their effectiveness (Pillai 2011). The uncertainty regarding predictors of success is an additional motivation behind HVTN’s adaptive efficacy trial design proposal, which allows for multiple parallel trials of different vaccine approaches with pre-planned interim analyses for the purpose of both rapidly discarding ineffective candidates and quickly identifying and advancing those showing promise (Corey 2011).
Inducing neutralising antibodies
Scientists continue to wrestle with the spiky problem of inducing antibodies that can effectively inhibit HIV. As described in last year’s report, several new broadly neutralising antibodies have been isolated from HIV positive individuals and their structures and targets are now being characterised in detail (Davenport 2011; Pancera 2010; Pejchal 2010; Zhou 2010). There has also been potentially significant progress in understanding how these rare antibodies are generated by the immune system. The production of antibodies by B cells involves a complex process called somatic hypermutation. Essentially, a B cell that is stimulated to make antibodies undergoes several rounds of division during which the genetic code for producing the antibody is shuffled each time, leading to alterations in the antibody structure. If the B cell’s genetic mutations produce an antibody with an improved ability to glom onto its target, the cell is selected to undergo more rounds of division. Repeated cycles of this mutation and selection process (referred to as ?affinity maturation?) lead to the generation of antibodies with a high affinity for their targets. Typically, affinity maturation takes an average of around 10-15 mutations. Remarkably, the broadly neutralising antibodies against HIV that have been identified show evidence of a more arduous affinity maturation process involving more than 60 mutations. The next step for vaccine researchers is to figure out whether this complex pathway can be recapitulated with a vaccine, leading to the generation of similarly effective antibodies. Signs so far are encouraging, but considerable work remains (Kwong 2011).
Table 2. PrEP and Microbicides Pipeline 2011
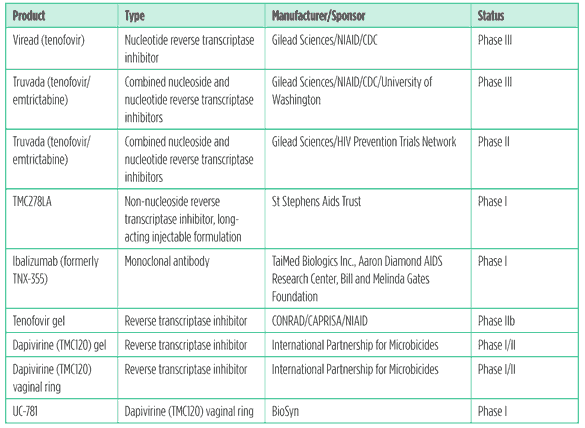
Preexposure prophylaxis
Preexposure prophylaxis (PrEP) is the prophylactic use of antiretroviral drugs to prevent HIV infection. In late 2010, the long-awaited first human PrEP efficacy results were announced and published in the New England Journal of Medicine (Grant 2010). The trial, named iPrEx, recruited 2,470 MSM and 29 transgender women at high risk of HIV infection, assigning them to receive daily Truvada (a combination pill containing the antiretrovirals tenofovir and emtrictabine) or placebo. Trial sites were located in Brazil, Ecuador,Peru,South Africa,Thailand,and the United States.Over an average of 1.2 years of follow up, the risk of acquiring HIV infection was reduced by 43.8% among participants in the Truvada arm compared to the placebo arm, a highly statistically significant result. There were a total of 36 infections in Truvada recipients compared to 64 in those on placebo. Additional follow up from May through August 2010 was reported in February of this year: the number of HIV infection endpoints increased to 48 vs. 83 for a final efficacy estimate of 42% (with a 95% confidence interval of 18-60%) (Grant 2011). Importantly, there was a strong correlation between adherence to the PrEP regimen and protection; a subset analysis of the Truvada arm comparing individuals with detectable drug levels to those without found that the presence of drug was associated with a greater than 90% reduction in HIV acquisition risk. However, this analysis also revealed that drug levels were undetectable in around half the participants assigned to Truvada, providing an indication that adhering to daily PrEP was problematic for a large proportion of the trial population.
In terms of tolerability, relatively few side effects were reported. Only nausea and unintentional weight loss of 5% or more were reported more frequently in the Truvada arm compared to placebo (in both cases, these side effects were noted by around 2% of Truvada recipients vs. 1% placebo). There were a total of five confirmed cases of elevated creatinine, a potential marker for kidney toxicity, all in the Truvada group. Four out of five of these individuals stopped and then restarted the drug without a recurrence of the problem. No other abnormal laboratory values were reported. No cases of drug resistance were observed in the participants who became HIV infected during the trial. However, there were three instances of resistance to emtrictabine documented among 10 people who were found to have had undetected, pre-seroconversion HIV infection at the time of study enrollment.
The iPrEx research team, led by Robert Grant at UCSF, now has funding from NIAID to conduct an open label evaluation (dubbed iPrEx OLk of Truvada as PrEP; all participants from the original randomised trial are being invited to participate. The goals for the study are to assess whether knowledge regarding Truvada’s efficacy has any effect on adherence and/or sex practices, and also to gather more safety data over a longer period of follow up.
The iPrEx data has generated considerable excitement in the PrEP field, but results are pending from trials being conducted in other populations. In a sobering development announced earlier this year, a trial of Truvada as PrEP at sites in Kenya, Malawi, South Africa, and Tanzania (the FEM-PrEP trial, sponsored by Family Health International) was stopped midstream after a review by the Data and Safety Monitoring Board (DSMB) found that it would not be able to show efficacy even if carried to completion.The DSMB decision was based on the observation that 56 HIV infections had occurred, evenly divided between the placebo and Truvada arms. The explanation for the FEM-PrEP outcome is as yet unclear.
The US Centers for Disease Control and Prevention (CDC) is sponsoring two ongoing PrEP efficacy trials: one is evaluating tenofovir (Viread) compared to placebo in 2,400 injection drug users in Thailand, the other is looking at Truvada in a population of 2,000 heterosexual men and women in Botswana. The University of Washington is comparing tenofovir to Truvada as PrEP in a trial involving 3,900 serodiscordant couples in Kenya and Uganda. The Microbicide Trial Network’s VOICE study has successfully completed enrolment of 5,000 African women and will compare three strategies: oral PrEP using tenofovir or Truvada and a tenofovir-containing vaginal microbicide gel. A recent DSMB review of VOICE gave it the green light to continue; follow up is due to end in June 2012 with results becoming available in early 2013.
The evidence from iPrEx regarding the difficulty of adhering to daily PrEP has renewed interest in intermittent dosing strategies. The HIV Prevention Trials Network (HPTN) is launching the ?ADAPT? study (Alternate Dosing to Augment PrEP Tablet-taking, also known as HPTN 067) which plans to compare different Truvada dosing schemes in 180 MSM and 180 heterosexual women at high-risk of acquiring HIV infection. The trial is not of sufficient size to evaluate efficacy but will compare tolerance, acceptability and drug levels.
Since the 2010 TAG pipeline report two novel PrEP agents have entered phase I trials:
- TMC278LA is a long-acting, injectable formulation of the approved antiretroviral drug rilpivirine that is being studied at four sites in the UK under the sponsorship of the St Stephens Aids Trust.
- Ibalizumab is a monoclonal antibody delivered via intermittent injection; it interferes with the interaction between HIV and the CD4 molecule, thereby inhibiting infection. Studies in people with HIV have documented significant viral load reductions (Bruno 2010). The phase I trial of ibalizumab as PrEP is unusual in that it is recruiting HIV negative volunteers at risk for HIV infection; normally, early-phase studies are restricted to participants with low or no risk of exposure to the virus.
Microbicides
Microbicides are substances that aim to prevent HIV infection via application to the vagina or rectum prior to (and in some cases also after) sex. Last year witnessed the first major microbicide breakthrough with the announcement of the results of CAPRISA 004, a phase IIb trial of tenofovir gel conducted in South Africa (Abdool Karim 2010). Women randomised to receive the gel had a statistically significant 39% reduction in risk of acquiring HIV infection. In raw numbers, there were 38 infections in the group of 445 tenofovir gel recipients and 60 among the 444 placebo recipients over an average of 18 months of follow up. The product was well tolerated and there was a strong association between drug levels in cervicovaginal fluid and protection from HIV (Kashuba 2010), echoing the findings from iPrEx and adding to the plausibility of the result.
Unexpectedly, CAPRISA 004 also showed that tenofovir gel offers significant protection against HSV-2 infection. Risk of acquiring HSV-2 was reduced by 51% (95% confidence interval: 30-78%) among women assigned to the active gel arm.This impressive finding suggests that tenofovir gel could have a dual impact on susceptibility to HIV, because HSV-2 infection is associated with an approximately 3-fold increase in relative risk of HIV acquisition in women (Freeman 2006).Tenofovir only inhibits HSV-2 at very high concentrations that cannot be achieved with oral dosing, but pharmacologist Angela Kashuba has shown that the gel form can reach sufficient levels in the genital tract (Kashuba 2010).
Since the initial presentation of the CAPRISA 004 results at the International AIDS Conference in Vienna in July 2010, the US Food and Drug Administration (FDA) has indicated that two additional confirmatory trials would provide sufficient data for the agency to consider the product for licensure. One trial, VOICE (described in the previous section), is ongoing. A second, called FACTS 001, has taken longer to secure funding than was anticipated, but is now expected to begin in South Africa in August 2011. The fate of a third tenofovir gel efficacy trial planned by the UK’s Microbicide Development Programme, MPD 302, is less certain.
Gilead Sciences has licensed the rights to produce tenofovir gel to the non-profit organization CONRAD, which is exploring options for manufacturing and marketing globally. CONRAD has recently announced that the South African government’s Technology Innovation Agency (TIA) will be granted the rights to manufacture and distribute tenofovir gel in Africa. TIA has, in turn, set up a joint venture called ProPreven consisting of TIA, Cipla Medpro and iThemba Pharmaceuticals. ProPreven will handle the registration, manufacturing and marketing of the gel if and when the data accrue to support licensure. A recent modeling study based on the results of CAPRISA 004 concluded that, over a twenty year period, the use of tenofovir gel in South Africa could avert up to two million new HIV infections and a million AIDS deaths (Williams 2011).
The next microbicide product that appears likely to undergo efficacy testing is a gel form of the nonnucleoside reverse transcriptase inhibitor drug dapirivine, which is being developed by the International Partnership for Microbicides (IPM). Phase I/II trials have shown that dapirivine gel can be safely delivered via a matrix intravaginal ring (Nel 2009), and IPM has ambitious plans to conduct two phase III efficacy trials of the approach involving a total of 6,000 women.
Table 3. Research toward a cure
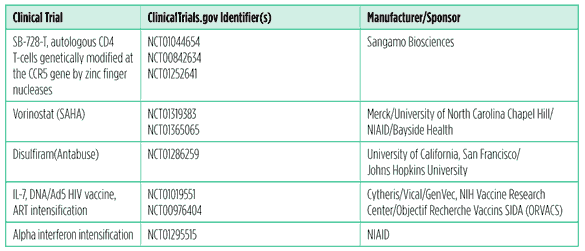
Not so long ago, prospects for an HIV cure were deemed so dim that even mentioning the word was generally frowned upon, lest it create false hopes. But it is important to appreciate that this semantic reticence did not equate to an absence of research; most of the trials and approaches included in the table above were in development long before the breakthrough case of Timothy Brown was reported. What Brown’s experience has done, however, is provide invaluable momentum for the research effort while at the same time bringing the possibility of a cure into the public consciousness. The elevated profile of the field has also spurred a flurry of review articles and opinion pieces in the scientific literature, delineating the challenges that lie ahead (Deeks 2010; Lafeuillade 2011; Lewin 2011a; Lewin 2011b; Margolis 2011; Siliciano 2010).
While the term ?cure research? is now increasingly invoked, it is not well defined. In terms of human trials, current strategies can be divided into three broad categories:
- Cell-protecting: approaches designed to protect potential target cells from HIV infection, e.g. via gene therapy.
- Reservoir-depleting: approaches that aim to reduce the amount of residual HIV that persists after viral replication is suppressed by ART.
- Immune-enhancing: approaches to bolster the immune response to HIV in hopes of enabling the body to control or even gradually eliminate residual viral reservoirs.
Sangamo Biosciences is pursuing a cell-protecting strategy based on a proprietary technology that allows targeting of specific genes. By pairing zinc finger proteins with enzymes called nucleases that can break up DNA, Sangamo’s approach disrupts the CCR5 gene and thus prevents expression of the CCR5 co-receptor on modified cells (Urnov 2010). In current trials, CD4 T cells are extracted from participants via apheresis, subjected to the zinc finger nuclease procedure in the laboratory, and then expanded in number and re-infused. Presentation of preliminary phase I results early in 2011 generated considerable excitement because the researchers were able to document significant CD4 T cell count increases and persistence of CCR5-deleted CD4 T cells at low but detectable levels in peripheral blood (Lalezari 2011). In a small subset of participants who underwent sampling from the gastrointestinal tract there was evidence that the majority of CD4 T cells in their gut were CCR5-deleted, suggesting that the modified cells had a particular survival advantage in this location, which is known to be a major site of HIV replication (Tebas 2011). Further results from these trials are eagerly anticipated. Unlike many cash-strapped biotech companies, Sangamo is better positioned to move its candidate HIV therapy through the pipeline due to a robust revenue stream obtained from licensing their gene modification technology for laboratory and agricultural use. Researchers are also collaborating with Sangamo to study the effects of CCR5-deleted stem cells in individuals with HIV who require stem cell transplants for AIDS-related lymphoma; the trial is not yet open for enrollment but is slated to take place at the City of Hope in Los Angeles (Cannon 2011).
While Sangamo ultimately has marketing ambitions for its gene therapy, the other examples of cure-related trials are more exploratory in nature. Laboratory experiments indicate that a class of anticancer drugs called HDAC inhibitors can activate the otherwise silent latent HIV reservoir and one such drug – vorinostat (SAHA) – is now being studied for this purpose in both the US and Australia (the principal investigators are David Margolis at the University of North Carolina and Sharon Lewin at Monash University, respectively). The downside of HDAC inhibitors is a daunting toxicity profile that has led these trials to proceed with extreme caution. The goal is not to develop vorinostat but rather to find out if HDAC inhibition can have measurable effects on the HIV reservoir in humans; a positive outcome would justify investment in the development of safer candidates with similar mechanisms of action. Two large pharmaceutical companies, Merck and Gilead, have publicly acknowledged having research programmes looking at HIV latency reversal and Merck is involved in the vorinostat trials for this reason.
Disulfiram (Antabuse) is an approved drug used to treat alcoholism, its HIV latency-reversing properties emerged from a large drug screening study conducted by the laboratory of Robert Siliciano at Johns Hopkins University (Xing 2011). The discovery is a testament to the impact of the recently formed amfAR Research Consortium for HIV Eradication (ARCHE), which funded the work of Siliciano and collaborator Steve Deeks at the University of California San Francisco. Deeks’ group is now conducing a small trial to investigate whether disulfiram has an effect on latent HIV reservoirs in vivo.
Objectif Recherche Vaccins SIDA (ORVACS) is a foundation based in France that was originally established to support therapeutic HIV vaccine research. ORVACS is sponsoring two trials, Eramune 01 and 02, that are investigating combination approaches to HIV reservoir reduction. Eramune 01 will look at intensifying standard antiretroviral therapy (ART) with the integrase inhibitor raltegravir and CCR5 inhibitor maraviroc, with or without the addition of the cytokine IL-7. Eramune 02 employs the same ART intensification, with or without the addition of a DNA/Ad5 prime-boost therapeutic vaccine developed by the Vaccine Research Center at the National Institutes of Health.
At the National Cancer Institute, an alternate means of ART intensification is being explored. Frank Malderelli’s research group is conducting a pilot study of the cytokine alpha interferon as an adjunct.The trial was motivated by an observation that individuals co-infected with HIV and hepatitis C may have declines in residual HIV viral load levels during alpha interferon treatment.
Although only a limited number of clinical trials can reasonably be described as cure-related at the current time, this is likely to rapidly expand. Plans are afoot at the AIDS Clinical Trials Group (ACTG) to investigate a PD-1 inhibitor made by Merck; this approach is intriguing as it may have the potential to both enhance the immune response to HIV and activate latent viral reservoirs (Kaufmann 2009; DaFonseca 2010). The company VIRxSYS has therapeutic vaccine candidate, VRX1273, that is on the verge of phase I; the construct is unusual in that it consists of a lentiviral vector based on HIV itself (Lemiale 2010). Many older gene therapies and therapeutic vaccines that remain in the pipeline (see Tables 4 and 5) could potentially fit under the new rubric of “cure-related” (and may eventually feature in trials for that purpose), because they aim to protect susceptible cells from HIV or improve immune responses to the virus.
If appropriate circumstances arise, researchers also intend to try and duplicate the case of Timothy Brown. This is a complex goal as it involves identifying people with HIV and cancer requiring stem cell transplantation, then finding a matched donor who lacks the CCR5 receptor (in genetic terms, a donor homozygous for the CCR5?32 mutation). The doctors involved in Brown’s case, led by clinician Gero H? are spearheading this ongoing effort (H?2011).
Immune-based & gene therapies
The developmental pathway for these types of candidate HIV therapies is particularly complex. Because of the effectiveness of antiretroviral drugs in treating HIV, IBTs and gene therapies needs to be able to supplement their effects, or replace them (either intermittently or permanently); the latter goal obviously overlaps with the idea of a ?functional cure? described in the previous section.
There are potential opportunities for supplementing ART because a proportion of HIV-positive individuals experience persistent immune dysfunction despite suppression of viral replication to undetectable levels. The features of this dysfunction typically include poor recovery of CD4 T cell numbers in peripheral blood, persistent skewing of the CD4:CD8 T cell ratio (usually around 2:1 in healthy individuals but often <1 in people with HIV), elevated immune activation and inflammation, decreased numbers of naive CD4 and CD8 T cells and increased numbers of dysfunctional, worn-out CD4 and CD8 T cells that are termed “senescent” (Deeks 2011; Erikstrup 2010; Fernandez 2006; Massanella 2010; Robbins 2009). The senescent cells resemble those that have been shown to accrue in very elderly individuals without HIV infection. The most significant risk factor for experiencing these persistent immunological perturbations on ART is initiating treatment at a low CD4 T cell count. Importantly, research shows that there is a link between these phenomena and an increased risk of illness and mortality (Kesselring 2011; Schechter 2006; Zoufaly 2011); therefore, therapies capable of enhancing the restoration of the immune system might be able to improve the prognosis for this subset of people with HIV. Currently the cytokine IL-7 appears to be the only IBT with any prospect of being evaluated for clinical benefit in this setting.There are however several other approaches that attempt to address different aspects of immune dysfunction, including anti-inflammatories and bone marrow stimulants.
Table 4. Immune-based & gene therapy pipeline 2011
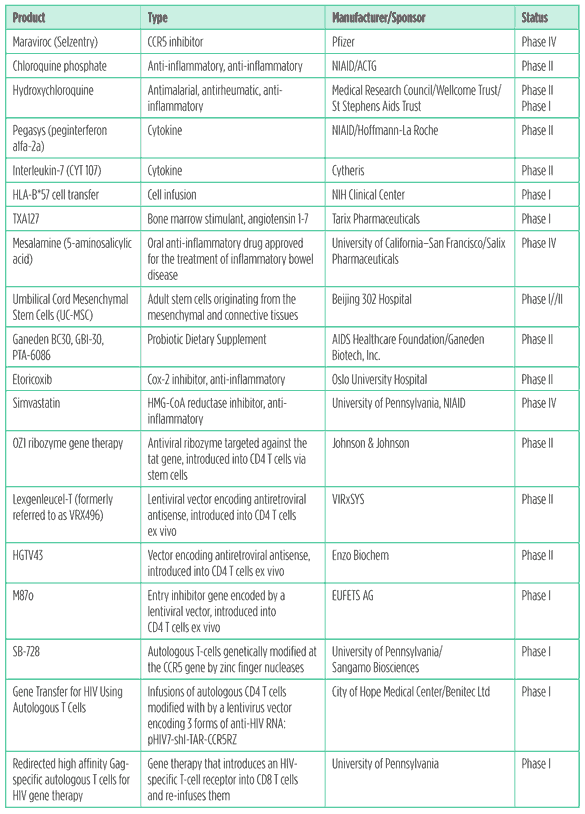
Anti-inflammatories
The antimalarial drugs chloroquine phosphate and hydroxychloroquine are being assessed for their potential to reduce immune activation and improve CD4 T cell recovery in individuals on ART. A very small pilot trial of chloroquine phosphate that was published last year reported significant reductions in markers of immune activation over two months of treatment (Murray 2010).
Mesalamine is an oral anti-inflammatory drug that acts particularly on the cells of the gut (Iacucci 2010), and the US Food and Drug Administration has approved it for the treatment of ulcerative colitis, proctitis, and proctosigmoiditis. The research group of Steve Deeks at the University of California San Francisco (UCSF) is conducting a small study to ascertain if mesalamine can reduce inflammation levels in HIV-positive people on ART. The study is motivated by evidence that leakage of normally friendly gut bacteria into systemic circulation (microbial translocation) contributes to immune activation in HIV infection (Brenchley 2006) and is associated with poor immune reconstitution on ART (Marchetti 2008). The same research group has also probed the contribution of CMV co-infection to immune activation in people on ART by conducting a trial of the anti-CMV drug valganciclovir. The study, now published, found that markers of activation on CD8 T cells were significantly reduced by this intervention, suggesting suppression of CMV replication could have benefits in co-infected people with HIV (Hunt 2011a). Unfortunately the toxicity profile of valganciclovir makes it a poor candidate for chronic use, so safer anti-CMV therapies will be needed in order for this potential lead to be followed.
A number of investigators are evaluating whether the approved CCR5 inhibitor maraviroc can dampen immune activation and enhance immune reconstitution. Results from two trials presented at the Conference on Retroviruses and Opportunistic Infections in 2011 were not particularly encouraging, however. In one uncontrolled, single-arm experiment markers of immune activation were reported decrease (Wilkin 2011), but in the other randomised placebo-controlled study these markers increased in blood and gut samples (Hunt 2011b). In neither case did CD4 T cell counts increase significantly.
Two new clinical trials are looking at the anti-inflammatory effects of the pain medication etoricoxib and the lipid lowering agent simvastatin in HIV, respectively. The etoricoxib trial is enrolling people naive to ART due to a prior study finding that the drug reduced immune activation and improved T cell function in individuals for whom ART was not indicated based on European guidelines (Pettersen 2011). Researchers at the University of Pennsylvania are recruiting individuals off ART for their study of simvastatin, in order to assess if the drug can reduce the monocyte inflammation and inflammatory cytokine production that has been linked to brain disease in HIV.
Cell infusion and gene therapies
In addition to being involved in the Sangamo Biosciences trials described in the section on research toward a cure, Carl June’s research group at the University of Pennsylvania is evaluating a different gene therapy that modifies CD8 T cells ex vivo, equipping them with a T cell receptor (TCR) that is particularly adept at recognising HIV-infected cells (Varela-Rohena 2008). The souped-up CD8 T cells are then expanded and re-infused back into the individual. The ultimate goal is to combine both CD4 and CD8 T cell gene therapy approaches in order to enhance the ability of both subsets to deal with HIV.
Last year, researcher John Rossi from City of Hope in Los Angeles published results from a phase I trial of a combined gene therapy approach in HIV-infected individuals undergoing hematopoietic stem cell (HSC) transplantation for AIDS-related lymphoma (DiGiusto 2010). Genes encoding three different anti-HIV RNA molecules were introduced into a subset of transplanted HSCs in four individuals, and long-term persistence in multiple cell lineages was demonstrated, albeit at very low levels. Although no therapeutic effect could be demonstrated, the study offers evidence that the concept is feasible. Rossi’s group is now collaborating with Paula Cannon at the University of California at Los Angeles (UCLA) and Sangamo Biosciences to study the deletion of the CCR5 gene in HSCs, in the same setting of AIDS-related lymphoma.
IL-7
The cytokine IL-7 plays a key role in supporting T-cell development and the proliferation and survival of naive and memory T-cells. Results from two phase I trials of IL-7 in people with HIV reported substantial increases in CD4 and CD8 T-cell counts even at the lowest dose (Levy 2009; Sereti 2009). The cytokine was well tolerated. A new glycosylated form of IL-7 that allows less frequent administration is currently in phase II trials. The manufacturer is a French company named Cytheris. The ACTG is considering the possibility of studying the clinical effects of IL-7 in individuals with poor CD4 recovery despite HIV suppression.
The ability of IL-7 to reduce HIV reservoirs is also under investigation, but there is debate regarding its potential in this setting; while viral load blips were observed in one phase I study (Sereti 2009), it has been argued that the source of this virus was not long-lived reservoirs (Imamichi 2011). Furthermore, it has been shown that under some circumstances IL-7 may expand the number of latently HIV-infected CD4 T cells by stimulating their division (Chomont 2009).
Table 5. Therapeutic vaccines pipeline 2011
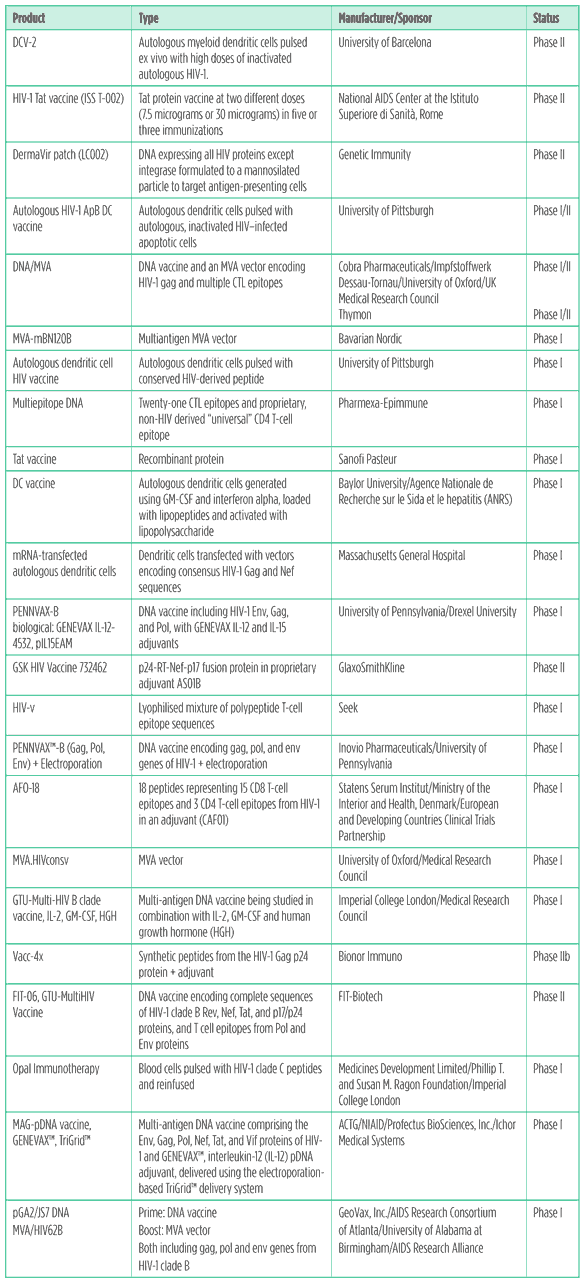
Therapeutic vaccines
The proposal that therapeutic vaccination might enhance the immune response to HIV was floated soon after the virus was first discovered. But clinical trials of a variety of candidates proved consistently disappointing, with no clear evidence of benefit. The most publicised was a large clinical endpoint study of Jonas Salk’s candidate, Remune, which showed no significant differences in health outcomes between vaccine and placebo (Khan 2000). The arrival of combination ART lessened the need for a therapeutic vaccine, but also opened up a window of opportunity because it became possible to try and induce new immune responses to HIV without interference from the potentially immune-suppressive effects of ongoing viral replication. An array of therapeutic vaccines are undergoing testing in this context.
Scientists at the University of Barcelona published the first data on their dendritic cell-based approach earlier this year (Garcia 2011). A small but statistically significant viral load reduction was observed in the vaccine recipients, along with some evidence for an inverse association between HIV-specific T cell responses and viral load. The company Argos Therapeutics is also developing a dendritic cell-based therapeutic vaccine, with the twist that it is ?personalised? by loading the cells with viral RNA from the person who is going to receive the vaccine; the goal is to induce immune responses that are exquisitely specific to each individual’s HIV infection (Routy 2010).
Italian researcher Barbara Ensoli at the National AIDS Center at the Istituto Superiore di Sanit?n Rome continues to plug away with studies of a therapeutic Tat protein vaccine that has been in development for over a decade now. Ensoli and colleagues took the dubious step of publishing interim results from an ongoing trial in people on ART, claiming a variety of beneficial effects associated with vaccination, including reductions in markers of immune activation (Ensoli 2010).
A novel approach to therapeutic immunization that recently entered human testing is Opal Immunotherapy. Developed by Stephen Kent’s research group at the University of Melbourne, it involves repurposing sets of overlapping peptides derived from HIV that are normally only used in laboratories to measure T cell responses against the virus. Kent had the idea to try and use the peptides as a vaccine by mixing them with either peripheral blood mononuclear cells (PBMC) or whole blood, then infusing this mixture. Studies in SIV-infected macaques have shown some promise (De Rose 2008) and a phase I trial is now underway.
An alternate strategy being pursued by some therapeutic vaccine manufacturers is immunization of HIV-positive people prior to any significant CD4 T-cell decline, with the aim of delaying the need for ART. At the 2010 International AIDS Conference, results from a 60-person randomised controlled study of this type were reported, showing that a DNA vaccine manufactured by FIT Biotech lowered viral load by around half a log after two years of follow up. A small but statistically significant increase in CD4 T cell counts was also observed (Vardas 2010).
The largest pharmaceutical company involved in this research area is GlaxoSmithKline. Their vaccine candidate, obscurely designated 732462, consists of a fusion protein including several HIV antigens (p24, p17, reverse transcriptase and Nef ) in a proprietary adjuvant, AS01B. GSK is conducting a phase II trial exploring the potential for immunization to delay the need for ART.
Conclusion
As incremental as it may be, there is no doubt that significant progress has occurred over the past few years. Until quite recently, there was no evidence of efficacy from any vaccine, microbicide, or PrEP trial. But the investment in research is starting to pay off, and while it may be frustrating that no product is yet available, there is definitely light at the end of these pipelines.
For cure research, the shift from the laboratory to clinical trials is only just beginning. But there is already hope in the form of Timothy Brown, and an increasing demand for science to push beyond the ART-for-life paradigm that currently prevails. The rising profile of cure research is also providing a welcome opportunity for immune-based and gene therapies to emerge from relative obscurity and enter the mainstream.
References
Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010 Sep 3;329(5996):1168-74. Epub 2010 Jul 19.
Allers K, H?G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. Evidence for the cure of HIV infection by CCR5?32/ ?32 stem cell transplantation. Blood. 2011 Mar 10;117(10):2791-9. Epub 2010 Dec 8.
Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365?71. Epub 2006 Nov 19.
Bruno CJ, Jacobson JM. Ibalizumab: an anti-CD4 monoclonal antibody for the treatment of HIV-1 infection. J Antimicrob Chemother. 2010 Sep;65(9):1839-41. Epub 2010 Jul 17.
Cannon P, Holt N, Hofer U, et al. CCR5 Knock-out in Hematopoietic Stem Cells. Paper #:164, 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, Feb 27-Mar 2, 2011
Cooper D, Wright KJ, Calderon PC, et al. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and g gene truncation reduces neurovirulence and enhances immunogenicity in mice. J Virol. 2008 Jan;82(1):207-19. Epub 2007 Oct 17.
Corey L, McElrath MJ. HIV vaccines: mosaic approach to virus diversity. Nat Med. 2010 Mar;16(3):268-70
Corey L, Nabel GJ, Dieffenbach C, Gilbert P, Haynes BF, Johnston M, Kublin J, Lane HC, Pantaleo G, Picker LJ, Fauci AS. HIV-1 Vaccines and Adaptive Trial Designs. Sci Transl Med. 2011 Apr 20;3(79):79ps13.
Currier J, de Souza M, Ratto-Kim S, et al. Induction of Cytolytic, V2-specific, Polyfunctional CD4+ T Cells in the Thai Phase III HIV Vaccine Trial. Paper # 331, 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, Feb 27- Mar 2, 2011
DaFonseca S, Chomont N, El Far M, Boulassel R, Routy J, S?ly RP: Purging the HIV-1 reservoir through the disruption of the PD-1 pathway [Abstract]. Journal of the International AIDS Society 2010, 13(Suppl 4):O15.
Davenport TM, Friend D, Ellingson K, et al. Binding interactions between soluble HIV envelope glycoproteins and quaternary-structure-specific MAbs PG9 and PG16. J Virol. 2011 May 4. [Epub ahead of print]
Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011 Feb 18;62:141-55.
De Rose R, Fernandez CS, Smith MZ, et al. Control of viremia and prevention of AIDS following immunotherapy of SIV-infected macaques with peptide-pulsed blood. PLoS Pathog. 2008 May 2;4(5):e1000055.
Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 201;7(1):4?10.
DiGiusto DL, Krishnan A, Li L, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2(36):36ra43.
Ensoli B, Bellino S, Tripiciano A, et al. Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T-cells and improves immune function in subjects on HAART. PLoS One. 2010 Nov 11;5(11):e13540.
Erikstrup C, Kronborg G, Lohse N, Ostrowski SR, Gerstoft J, Ullum H. T-cell dysfunction in HIV-1-infected patients with impaired recovery of CD4 cells despite suppression of viral replication. J Acquir Immune Defic Syndr. 2010 Mar 1;53(3):303-10.
Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006 Aug;120(2):163-70. Epub 2006 Jun 9.
Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006 Jan 2;20(1):73-83. Review.
Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174(11):6571?76.
Garc?F, Climent N, Assoumou L, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis. 2011 Feb 15;203(4):473-8. Epub 2011 Jan 13.
Gilbert PB, Berger JO, Stablein D, et al. Statistical interpretation of the RV144 HIV vaccine efficacy trial in Thailand: a case study for statistical issues in efficacy trials. J Infect Dis. 2011 Apr 1;203(7):969-75.
Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587-99. Epub 2010 Nov 23.
Grant RM, Lama JR, Glidden D, and iPrEx Study Team. Pre-exposure Chemprophylaxis for Prevention of HIV among Trans-women and MSM: iPREx Study. Paper #92, 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, Feb 27- Mar 2, 2011
Hansen SG, Ford JC, Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011 May 26;473(7348):523-7. Epub 2011 May 11.
Hansen SG, Powers CJ, Richards R, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010 Apr 2;328(5974):102-6.
Hersperger AR, Pereyra F, Nason M, Det al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6(5):e1000917.
Hunt P, Shulman N, Hayes T, et al. Immunomodulatory Effects of MVC Intensification in HIV-infected Individuals with Incomplete CD4+ T Cell Recovery during Suppressive ART. Paper#153LB, 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, Feb 27- Mar 2, 2011
Preventive Technologies, Immune-Based and Gene Therapies, and Research Towards A Cure
H?G, Thiel E. Allogeneic transplantation of CCR5-deficient progenitor cells in a patient with HIV infection: an update after 3 years and the search for patient no. 2. AIDS. 2011 Jan 14;25(2):273-4.
Iacucci M, de Silva S, Ghosh S. Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol. 2010;24(2):127?33.
Kahn JO, Cherng DW, Mayer K, Murray H, Lagakos S. Evaluation of HIV-1 immunogen, an immunologic modifier, administered to patients infected with HIV having 300 to 549 x 10(6)/L CD4 cell counts: A randomised controlled trial. JAMA. 2000 Nov 1;284(17):2193- 202. Erratum in: JAMA 2001 May 2;285(17):2197.
Kashuba AD, Abdool Karim SS, Kraft E et al. Do systemic and genital tract tenofovir concentrations predict HIV seroconversion in the CAPRISA 004 tenofovir gel trial? Abstract#TUSS0503, XVIII International AIDS Conference, Vienna, July 18-23, 2010.
Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009 May 15;182(10):5891-7. Review.
Kesselring A, Gras L, Smit C, et al. Immunodeficiency as a Risk Factor for Non-AIDS-Defining Malignancies in HIV-1-Infected Patients Receiving Combination Antiretroviral Therapy. Clin Infect Dis. 2011 Jun;52(12):1458-65.
Kim J. The Search for Antibody Correlates of Protection for HIV-1 Acquisition in RV144: An Update. Paper #65, 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, Feb 27- Mar 2, 2011
Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203.
Kwong, PD. Prospects for Generating VRC01-Like Antibodies Revealed by Crystal Structures and 454 Pyrosequencing. Paper #016, Keystone HIV Evolution, Genomics and Pathogenesis and Protection from HIV: Targeted Intervention Strategies (X8), Whistler, British Columbia, Canada, March 20?25, 2011
Lai L, Kwa S, Kozlowski PA, et al. Prevention of Infection by a Granulocyte-Macrophage Colony-Stimulating Factor Co-Expressing DNA/Modified Vaccinia Ankara Simian Immunodeficiency Virus Vaccine. J Infect Dis. 2011 Jul;204(1):164-73.
Lalezari J, Mitsuyasu R, Deeks S et al. Successful and Persistent Engraftment of ZFN-M-R5-D Autologous CD4 T Cells (SB-728-T) in Aviremic HIV-infected Subjects on HAART. Paper # 46, 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, Feb 27- Mar 2, 2011
Lehner T, Wang Y, Whittall T, McGowan E, Kelly CG, Singh M. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans. 2004 Aug;32(Pt 4):629-32.
Lemiale F, Asefa B, Ye D, Chen C, Korokhov N, Humeau L. An HIV-based lentiviral vector as HIV vaccine candidate: Immunogenic characterization. Vaccine. 2010 Feb 23;28(8):1952-61.
L?urneau S, Im EJ, Mashishi T et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One. 2007 Oct 3;2(10):e984.
Levy Y, Lacabaratz C, Weiss L, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119(4):997?1007. Epub 2009 Mar 16.
Li JZ, Brumme ZL, Brumme CJ, et al. Factors associated with viral rebound in HIV-1-infected individuals enrolled in a therapeutic HIV-1 gag vaccine trial. J Infect Dis. 2011 Apr 1;203(7):976-83.
Lorin C, Mollet L, Delebecque F, et al. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralising antibodies and cellular immune responses to HIV. J Virol. 2004 Jan;78(1):146-57.
Marchetti G, Bellistr?M, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22(15):2035?8.
Marin B, Thi?ut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23(13):1743?53.
Massanella M, Negredo E, P?z-Alvarez N, et al. CD4 T-cell hyperactivation and susceptibility to cell death determine poor CD4 T-cell recovery during suppressive HAART. AIDS. 2010 Apr 24;24(7):959-68.
Mitsuyasu RT, Merigan TC, Carr A, et al. Phase II gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15(3):285?92. Epub 2009 Feb 15.
Murray SM, Down CM, Boulware DR, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection.J Virol. 2010 Nov;84(22):12082-6. Epub 2010 Sep 15.
Napolitano LA, Schmidt D, Gotway MB, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118(3):1085?98.
Nel A, Smythe S, Young K, Malcolm K, McCoy C, Rosenberg Z, Romano J. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51(4):416?23.
Pancera M, McLellan JS, Wu X, et al. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralise HIV-1. J Virol. 2010 Aug;84(16):8098-110. Epub 2010 Jun 10.
Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011 May;157(2):175-9. Epub 2010 Oct 1.
Pejchal R, Walker LM, Stanfield RL, et al. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11483-8. Epub 2010 Jun 2.
Pettersen FO, Torheim EA, Dahm AE, et al. An Exploratory Trial of Cyclooxygenase Type 2 Inhibitor in HIV-1 Infection: Downregulated Immune Activation and Improved T Cell-Dependent Vaccine Responses. J Virol. 2011 Jul;85(13):6557-66. Epub 2011 Apr 13.
Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209?20. Epub 2009 Oct 20.
Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009 Feb 1;48(3):350-61.
Rodger AJ, Fox Z, Lundgren JD, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200(6):973?83.
Rodgers KE, Oliver J, di Zerega GS. Phase I/II dose escalation study of angiotensin 1-7 [A(1-7)] administered before and after chemotherapy in patients with newly diagnosed breast cancer. Cancer Chemother Pharmacol. 2006;57(5):559?68. Epub 2005 Aug 12.
Rolland M, Tovanabutra S, deCamp AC, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011 Mar;17(3):366-71. Epub 2011 Feb 27.
Rosario M, Bridgeman A, Quakkelaar ED, et al. Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. Eur J Immunol. 2010 Jul;40(7):1973-84.
Routy JP, Nicolette C. Arcelis AGS-004 dendritic cell-based immunotherapy for HIV infection. Immunotherapy. 2010 Jul;2(4):467-76.
Schechter M, Tuboi SH. Discordant immunological and virological responses to antiretroviral therapy. J Antimicrob Chemother. 2006 Sep;58(3):506-10. Epub 2006 Jul 19.
Sereti I, Dunham RM, Spritzler J, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113(25):6304?14. Epub 2009 Apr 20.
Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011 Feb 17;6(2):e16103.
Smith K, Zheng L, Bosch R, et al. Treatment with recombinant growth hormone is associated with modest improvement in CD4 lymphocyte reconstitution in HIV-infected persons on antiretroviral therapy: results of ACTG A5174. AIDS Res Hum Retroviruses. 2010;26(4):425?32.
Stassen FR, Vega-C
