Pipeline report 2021: HIV drugs in development
17 September 2021. Related: Special reports, Supplements, Pipeline report, Antiretrovirals.
 Simon Collins, HIV i-Base
Simon Collins, HIV i-Base
For all the focus and difficulties over COVID-19, this year is still an exciting year for HIV pipeline research.
This annual review, produced as an i-Base supplement, references 120 papers on key developments over the last 14 months.
It is updated in real time to include important developments during the next year, until the next full report.
In addition to this single web page version, this report will be available as different PDF files.
- The full version includes more information for each drug, with full references. This PDF does not include updates after August 2021.
Download full report Aug 2021 – PDF (500 Kb) - A “Pipeline-lite” version with reduced summaries and references will be produced next month for the i-Base Fit For Purpose Report.This is still in press.
- Russian translation (Aug 2021) – PDF
A postscript update is included as section 11 at the end of the report. This is for important developments over the next year until the next version of the pipeline review.
Abbreviations in this report
CROI: Conference on Retroviruses and OIs.
EMA: European Medicines Agency.
FDA: Federal Drug Administration (USA).
IAS: International AIDS Society.
MDR: Multidrug Resistance.
MHRA: Medicines and Healthcare Products Regulatory Agency (UK).
1. Introduction: pipeline 2021 after COVID-19
This report reviews developments over the last 14 months since the last edition produced for CROI 2020. [1]
It is also updated to include important results presented at the IAS 2021 virtual conference.
The HIV pipeline this year is exciting and significant.
The three main research-based drug companies – Gilead Sciences, Merck/MSD and ViiV Healthcare – are all focused on simplified ART. All have current or planned dual regimens supported by the greater potency of recent drugs. These companies also have long-acting compounds in new drug classes. If effective, within a few years, we could have many alternatives to daily oral ART.
This is also a unique time because many of the pipeline compounds have the potential to be used for both treatment and prevention. As well as this, for the first time in many years, much of the current pipeline comes from new drug classes that could be used for people who are treatment-naive, treatment-experienced, and with multidrug resistant (MDR) HIV.
The same companies developing new drugs for treatment and prevention are also investing in strategies to cure HIV.
Now, the research challenge is not only to produce more effective and tolerable treatments – and this bar is already set high – but to make sure that these are affordable. And that the participants in registrational studies reflect the demographics of the global HIV population.
A systematic review of demographics in industry studies was presented at CROI this year from the US-based community organisation AIDS Treatment Activists Coalition (ATAC). [2]
The group looked at 146 phase 2, 3 and 4 studies and observational cohorts from 2010 to 2020. Men were still overrepresented, ethnicity/race-specific data was unreported for 65% of studies and this was suboptimal when reported. There was also little geographic diversity as 75% of studies were in the US. Few studies specified transgender participants and by including upper age limits mostly excluded older people.
This report was difficult to compile this year. Some HIV studies were delayed or stopped because of COVID-19. Other developments were harder to track because of the move to virtual meetings.
Nevertheless, this is an exciting time. The 2021 pipeline report includes updates on the following drugs:
Long acting cabotegravir and rilpivirine, fostemsavir, islatravir, MK-8507 (NNRTI), lenacapavir (capsid inhibitor), GSK3640254 (maturation inhibitor) and limited information on several bNAbs.
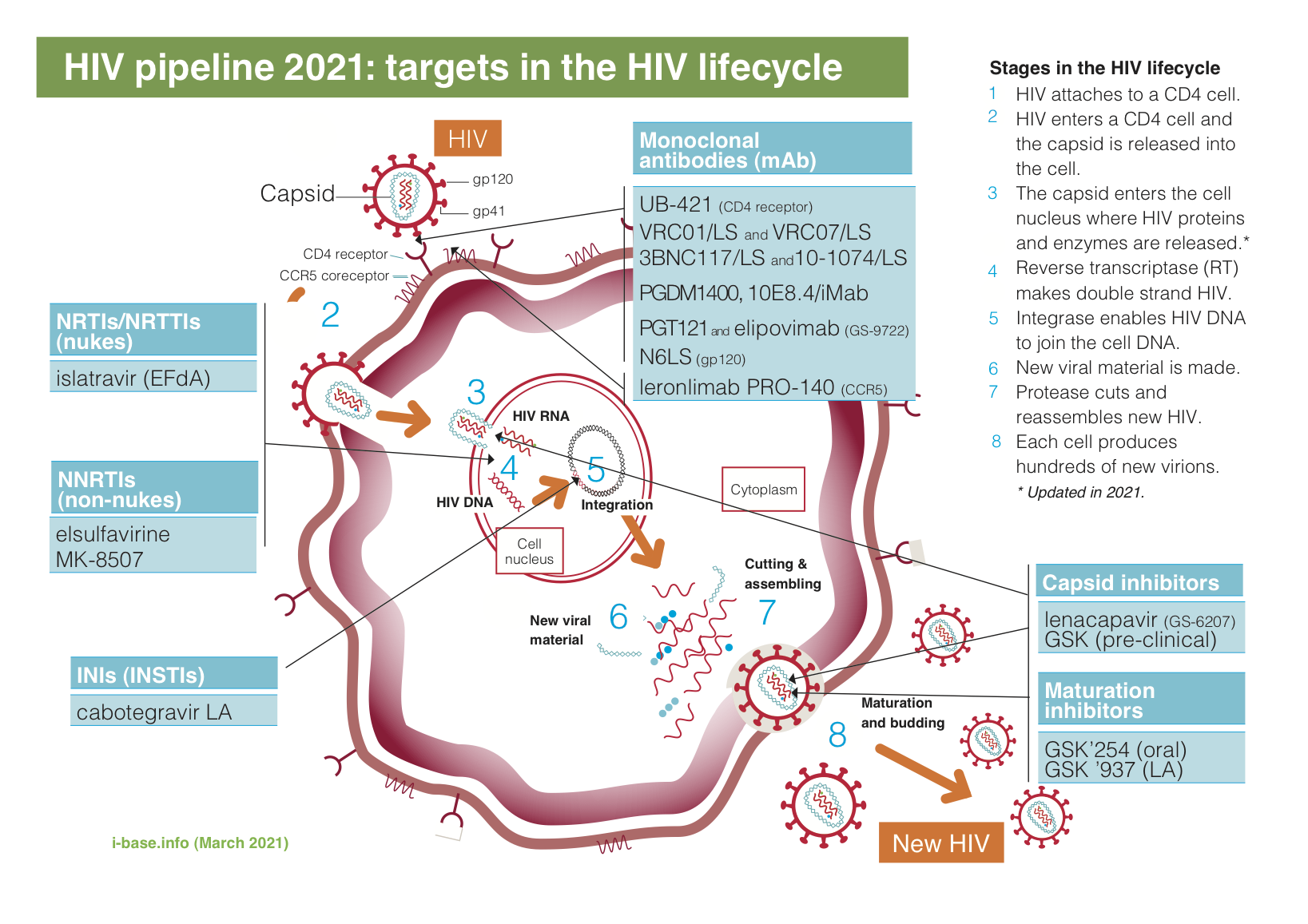
This years’ report also updates the viral lifecycle to show capsid uncoating in the cell nucleus rather than the cytoplasm. The ongoing debate over several years between different research groups seems to have been settled at CROI 2021 with convincing electron microscopy. Recommended viewing. [3]
Figure 1: HIV pipeline 2021: targets in the HIV lifecycle
Key: INSTI: integrase strand transfer inhibitor; LA: long-acting; mAb: monoclonal antibody; NRTI: nucleoside/tide reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor.
2. Update to the viral lifecycle
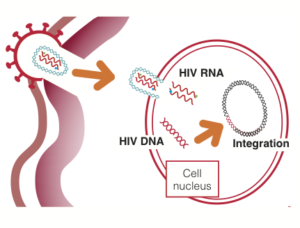
Fig 2. HIV capsid uncoats in CD4 cell nucleus.
For several years there has been an ongoing scientific debate about the timing and likely location for the uncoating of the viral capsid.
This now seems to have settled on being a late stage event, overturning decades of belief that reverse transcriptase converts HIV RNA to DNA in the cytoplasm.
Several studies at CROI 2021 showed that HIV capsid remains intact until entering the CD4 cell nucleus, including in a plenary lecture with impressive electron microscopy animations. [1, 2, 3] See Figure 2.
Radio-labelled proteins showed the capsid journey in real-time, slipping through pores in the nuclear core complex, narrow end first, with nanometres to spare.
This seemed a good time to update our diagrams on the viral lifecycle.
3. Summary over the last year
Main news over the last year included:
- Approval of cabotegravir/rilpivirine LA in EU and US in January 2021, although the NHS process for access is still ongoing.
- Approval of fostemsavir for HIV MDR in EU and US – in January 2021 and July 2020 respectively.
- Phase 2/3 results using islatravir (Merck/MSD), including in dual ART with doravirine. Pharmacokinetic (PK) data supporting a single monthly pill for use as PrEP.
- Once-weekly NNRTI MK-8507 is now in phase 2 studies in combination with weekly islatravir. (Note: development of MK-8507 was stopped in November 2021 due to mean dose-related reductions in CD4 and total lymphocyte counts). [ISL 19].
- Early results were presented for oral and subcutaneous formulations of maturation inhibitor (GSK-254) from ViiV and both treatment-naïve and MDR treatment.
- Gilead and Merck/MSD announced a new partnership for developing long-acting formulations.
- Long-acting capsid inhibitor lenacapavir has clinical results in naive and drug resistance, plus animal data for PrEP. Lenacapavir is given every 6 months.
- Limited phase 3 results on albuvirtide, given as a weekly injection.
- bNABs: developments include results from AMP studies using VRC01 as prevention. Numerous other studies on bNAbs used in combinations in prevention and cure-related studies.
- Combinectin, MK-8504 and MK-8583 were discontinued.
4. Update from IAS 2021 conference
 Although much of this report covers advances over the last year, the IAS 2021 conference included new data on many pipeline compounds.
Although much of this report covers advances over the last year, the IAS 2021 conference included new data on many pipeline compounds.
This included studies on long-acting cabotegravir/rilpivirine, fostemsavir, lenacapavir (as treatment and PrEP), islatravir (as treatment and PrEP) and albuvirtide.
- Several implementation studies showed that practical aspects of prescribing long-acting injectable ART can be overcome.
- Potential baseline resistance to cabotegravir/rilpivirine LA in a large French database reporting that 7% of >4000 treatment naive samples showed resistance to rilpivirine.
- Two posters on fostemsavir: on side effects out to 96 weeks and drugs in the background regimen of the BRIGHTE study.
- Efficacy and safety data on paediatric dolutegravir from the large international ODYSSEY study in treatment-naive and -experienced children.
- 96-week phase 2 safety data on islatravir/doravirine dual ART, including bone and kidney results, and use in renal disease.
- Islatravir PrEP studies included PK data easily supporting once-monthly oral pill and plans to include islatravir in a vaginal ring (combined with a contraceptive).
- Two PK posters on GS-8507 showing no drug interactions with either islatravir or oral contraceptives. Note: development of compound was stopped in November 2021).
- bNAbs: use in cure related research, susceptibility in the AMP studies,
- Albuvirtide phase 3 results: dual ART with lopinavir
5. Recent approvals and submitted applications
Several drugs were approved over the last year and lenacapavir was submitted to the FDA for MDR HIV. See Table 1.
Table 1: Recent regulatory approvals and submissions
| Compound/ formulation | Class | Approved / submitted |
Company |
| cabotegravir LA and rilpivirine LA injections | INSTI + NNRTI injections. | Approved US: January 2021.
Approved EU: January 2021. |
ViiV Healthcare Janssen |
| fostemsavir | gp120 attachment inhibitor. | Approved US: July 2020.
Approved EU: January 2021. |
ViiV Healthcare |
| paediatric dolutegravir | integrase inhibitor. | Approved US: June 2020.
Approved EU: November 2020. |
ViiV Healthcare |
| lenacapavir | capsid inhibitor for MDR HIV. | Submitted US: July 2021.
Submitted EU: August 2021. |
Gilead Sciences |
Cabotegravir/rilpivirine long acting injectable combination (CAB/RPV LA)
After a high-profile development, the long-acting injectable dual combination of cabotegravir and rilpivirine was approved by both the FDA and the EMA. The indication is as a switch option for people who are undetectable on current ART.
On 21 December 2020, EMA approval included the option for two-monthly dosing. Each formulation is also marketed with separate trade names: cabotegravir (Vocabria) and rilpivirine (Rekambys). [1]
The option of two-monthly dosing was based on results from the ATLAS-2M study using a higher 600/900 dose throughout (and now with follow-up to 96 weeks). Both studies used oral formulations for the first month. [2, 6]
In January 2021 this dual combination was approved by the US FDA, with an indication only for monthly injections. Both drugs are packaged and marketed together in the US with the trade name Cabenuva. [3]
However, on 24 February 2021, ViiV submitted an application to the FDA to expand the indication for two-monthly injections. [4]
Both regulatory approvals were based on results from the international FLAIR and ATLAS phase 3 studies (with >1200 participant combined). Approximated 25% of participants were women and 70% were white in the combined studies. Viral efficacy was >95% with approximately 1-2% meeting criteria for protocol defined viral failure at week 48. Continued follow-up has now been reported from both studies out to week 96. [5, 6]
Tolerability is generally very good, with injection site reactions being common, but also mild and not leading to discontinuation. Participants reported benefits on quality of life, but these results were from people who self-selected to use injectable treatment.
These long-acting formulations also raise new practical factors including adherence, missed dosing, supply issues: detectable drug levels have been reported out to over three years from a single dose. They also include challenges for health systems to move to injectable delivery.
CROI 2021 also included results about different aspects of CAB/RPV LA. [7–13]
- Improved renal and bone markers (when switching from TDF-based ART). [7, 8]
- Safety with hepatic impairment. [9]
- Similar efficacy results in older adults (above vs below 50 years old). [10]
- Population PK modeling for strategies for missed or late doses. [11, 12]
- Limited data on median weight and lipid changes were not significantly different to control groups at week 48. [13]
Week 124 results from the FLAIR study were presented at Glasgow 2020 and IAS 2021. These results also included limited data from participants not using an oral lead-in dosing for the first month. [14, 15]
However, a French study looking at a large drug resistance database (>4200 samples from 2010 to 2020 with both integrase and NNRTI sequences) reported that approximately 7% of treatment-naive people might have transmitted mutations to rilpivirine, especially in people with HIV sub-type A. Resistance to cabotegravir was <1.0 %. The study emphasised the need for baseline genotypic resistance test to detect polymorphisms, transmitted drug resistance and to define HIV-1 subtype. [16]
For additional details please refer to full prescribing information. [17]
Cabotegravir is also being studied as PrEP, with recent phase 3 HPTN 083 and HPTN 084 studies reporting superiority compared to daily oral TDF/FTC in trans women and gay men. [18, 19]
Fostemsavir
Approval of the gp-120 attachment inhibitor fostemsavir for HIV MDR in the US and EU – in July 2020 and January 2021 respectively. [1, 2]
This approval was based on 96-week results from the international BRIGHTE study in people with multidrug resistance, reported at IAS in 2019. [3] Which includes results in 272 participants in the randomised study and 99 participants using open-label fostemsavir. Further 96-week analyses were presented at IAS 2021, including new analyses of side effects and the diversity of treatments used in background ART – although this primarily included twice-daily dolutegravir. [4, 5]
As a drug in a new class, supported by results from BRIGHTE, fostemsavir can be a life-saving option for the small percentage of people with MDR HIV.
Fostemsavir is a twice-daily oral medication. For full details please see the product characteristics. [6]
A long-acting/extended release formulation is also being studied in a phase 1 study. [7]
Paediatric formulationsof dolutegravir
New paediatric formulations of dolutegravir were also approved this year which will dramatically improve treatment options for children globally.
On 12 June 2020 the US Food and Drug Administration (FDA) approval dolutegravir (Tivicay) tablets and dolutegravir tablets for oral suspension (Tivicay PD) for infants and children in combination with other antiretrovirals. [1]
Approval was granted to the originator manufacturer, ViiV Healthcare. The new formulations are indicated for paediatric patients at least 4 weeks old and weighing at least 3 kg who are ART-naive or ART-experienced but have not previously received an integrase strand transferase inhibitor (INSTI).
On 12 November, EMA gave a positive opinion recommending marketing authorisation for dolutegravir 5 mg dispersible tablets for young children with HIV. [2]
Several presentations at IAS 2021 included additional results from large international ODYSSEY study – from an additional cohort of 85 infants and children <14 kg. This included good 36-week efficacy results for younger children but also reported four cases of drug resistance in the main ODYSSEY study in the older age group – showing the importance of access to pipeline ART. [3, 4]
More comprehensive results were also presented at the paediatric workshop held just before IAS. [5]
Safety data from ODYSSEY in the main study was also generally good but included vulnerability of some participants to CNS side effects and mood changes. [6. 7. 8]
For more details please see the paediatric pipeline report. [9]
Lenacapavir – for MDR HIV
On 28 June 2021, lenacapavir was submitted to the US FDA as a treatment for MDR HIV (and to the EMA in August 2021). [1, 2]
This is even though lenacapavir is still only in phase 2 studies for treatment.
Although there are currently very few people with MDR HIV in high-income countries, the application did not use orphan status. This is because lenacapavir will also be submitted later for treatment-naive and -experienced populations.
The MDR submission was based on results from the phase 2/3 CAPELLA study in 72 highly treatment-experienced participants who had HIV multidrug resistance (MDR) to at least three drug classes. [3, 4]
Half the participants were randomised (2:1) to lenacapavir or placebo for 14 days (before optimising treatment) and half used open label lenacapavir. The median (range) change in viral load was –2.0 log copies/mL (range: –3.29 to –0.29) vs –0.08 (–1.93 to +0.31) in the active vs placebo groups.
Clinical results at week 26 from the 36 participants in the randomised section of this study were also presented at IAS 2021. [5]
Median age was 52 years (range: 23 to 78), 25% were women, 38% were back and 28% Hispanic/Lantinx.
Participants had been living with HIV for an estimated median of 24 years (range: 9 to 44 years). Extensive drug resistance to >2 drugs in each class at baseline was: 99% (NRTIs), 97% (NNRTIs), 81% (PIs) and 69% (INSTIs).
Virological results included 81% (n=29/36) of participants reaching an undetectable viral load (<50 copies/mL) and 89% (32/36) <200 copies/mL. There were no missing data with 7 and 4 participants having viral load >50 and >200 copies/mL, respectively. Although numbers are small, 4/6 participants with no active background drugs also reached <50 copies/mL.
The mean CD4 increase to 81 cells/mm3 included increases to >50 cells/mm3 in the 8/36 participants who had CD4 counts <50 cells/mm3 at baseline.
Limited data were available for the 11 participants who met criteria for resistance testing. Of these, 4/11 developed emerging mutations associated with drug resistance to lenacapavir: M66I (4), Q67H (1), K70N/R/S (1) and N74D (1) although related phenotypic impact was not discussed. Of these, 3/4 later suppressed, one with OBR change and two without. One person without other sensitive drugs who did not become undetectable reported a –1.7 log reduction in viral load. Although no new resistant mutations were reported for other ART, this is likely related to the relatively short follow-up.
Tolerability was good with no study discontinuations and no serious drug-related side effects. Injection site reactions were common (56%; 40/72) but mainly grade 1 (28/40) that resolved in a few days. None were grade 4 and the two grade 3 reactions resolved by days 4 and 8.
All participants have since received a second 6-monthly injection.
The application to the EMA in Europe was submitted in August 2021, with final decisions expected to take a year. [2]
Submission to the MHRA in the UK is likely to follow the EU decision. Over this extended period, a limited programme will hopefully enable access for individuals in critical need, although details have not yet been released.
For more details, see main section on lenacapavir below.
6. Drugs in phase 2/3 development
Table 2: HIV pipeline compounds by development phase (excluding individual bNAbs)
| Compound/ Company | Class | Notes | Phase |
| islatravir
(EFdA) Merck/MSD |
NRTTI | Highly potent, low dose, active against NRTI resistance. Long half-life, potential as oral (dosed daily, weekly and perhaps monthly) and an implant (annual). Monthly and annual formulations are for PrEP. | Phase 2/3 |
| islatravir / 3TC / doravirine
Merck/MSD |
FDC: NRTTI + NRTI + NNRTI |
FDC with generic 3TC and NNRTI doravirine. Current studies used triple combination for initial ART and switch to islatravir/doravirine for dual maintenance ART. | Phase 3 |
| islatravir / doravirine
Merck/MSD |
FDC: NRTTI + NNRTI | Dual FDC with NNRTI doravirine. Currently studies look at a switch option after viral suppression with triple drug ART. | Phase 3 |
| MK-8507
Merck/MSD |
NNRTI | New NNTRI being studied with weekly dosing of islatravir. | Phase 2 (Development on hold from November 2021). [ISL 19]. |
| Lenacapavir
Gilead |
capsid inhibitor | Activity at multiple stages of viral lifecycle. Sub-cutaneous injection every six months. Phase 3 data in MDR presented at CROI 2021 and IAS 2021. Phase 2 results in naive at IAS 2021. Phase 3 PrEP studies just starting. | Phase 2 and 3 |
| Maturation inhibitors: GSK3640254 and GSK3739937 | maturation inhibitor | Maturation inhibitor in phase 2 studies. | Phase 2 |
| albuvirtide | fusions inhibitor | Similar to enfuvirtide (T-20). Long-acting weekly formualtion. Already approved in China but phase 3 data only recently reported at IAS 2021. Developed by Frontier Biotechnologies. US studied listed with bNAb for MDR HIV. | Phase 3 |
| bNAbs: leronlimab, UB-421, VRC01, 3BNC117, 10-1074,10E8, N6, PGDM1400 and PGT121 etc. | bNAb’s:
Multiple targets include CD4 binding, v3 loop etc |
Many bNAbs are in development for prevention, treatment and cure research – often in long-acting LS formulations and in dual or triple combinations.
Many public and private research institutes (NIH, Rockefeller etc) and pharmaceutical research companies (Gilead, ViiV etc). See Table 3. |
Preclincal, Phase 1 to 3 |
Islatravir
Islatravir is an NRTI (technically, a nucleoside reverse transcriptase translocation inhibitor or NRTTI) derived from flavouring for soy sauce that hovered as a pipeline compound for several years (as EFdA) before being acquired by Merck/MSD in 2012. [1]
It is one of the most potent antiretrovirals with activity from oral dosing for treatment of 0.75 mg daily and 10 mg weekly. Current studies also include use for prevention with PrEP using 60 mg oral monthly dosing (12 pills a year) and an annual implant. Islatravir is also has the potential for a single oral dose as PEP.
The treatment programme is now focused on dual therapy with NNRTIs doravirine using daily dosing and with MK-8507 using weekly dosing. (See comment below on discontinuing development of MK-8507). [18]
Islatravir plus doravirine
CROI 2021 included week 96 results from a phase 2b study using islatravir and doravirine dual therapy (after dropping lamivudine at week 20). [2]
This study presented an analysis of viral load blips >50 copies/mL in participants who then became undetectable again without changing treatment. Blips were reported more frequently in the control arm (DOR/3TC/TDF) compared to the dual therapy group (DOR/ISL). There were 8 blips in 7/86 (8.1%) vs 4 blips in 4/28 (14.3%) in the combined ISL vs control groups respectively. Baseline VL was >100,000 copies/mL in 7/8 vs 0/4 of the ISL and control groups respectively.
Ongoing studies include in treatment-naive, experienced, switch, MDR and paediatric populations. [3–8]
Islatravir plus MK-8507 – and MK-8507
Note: Development of MK-8705 was put on hold in November 2021 due to mean dose-related decreases in CD4 and total lymphocyte count when dosed weekly with islatravir. Mean reductions have also been reported when islatravir is not used with MK-8507, including when used as PrEP in HIV negative studies. However, islatravir studies currently will continue with additional monitoring. [18]
Islatravir is also being studied in combination with the NNRTI MK-8507 as a weekly oral combination.
At CROI 2021, a single 20 mg once-weekly dose of islatravir produced intracellular concentrations similar to steady state using the 0.75 mg daily dose. After 14 days, islatravir levels were still five-fold above the inhibitory quotient (IQ) for lamivudine-resistant HIV, showing this would also have some flexibility if a single dose was late or missed. [9]
Two studies at Glasgow 2020 presented results on MK-8507. [10]
This compound has a half-life of ~70 hours and mean viral load reductions of –1.5 log were reported one week after a single dose, in a phase 2 study in 18 white men.
The study modelling for dosing MK-8507 was based on real world simulations in combination with islatravir and assuming 80% adherence. The study reported that the three doses studied – 100 mg, 200 mg and 400 mg – should all provide >90% efficacy against common NNRTI mutations including K103N and Y181C.
An oral presentation at CROI 2021 also presented additional new information about MK-8507, including activity against early NNRTIs (K103N, Y181C and G190A) – with a resistance profile similar to doravirine. [11]
Two PK studies on MK-8507 at IAS 2021 reported no drug interactions with either islatravir or oral contraceptives. [12, 13]
Islatravir as PrEP
A PK/PD analysis at CROI 2021 defined the islatravir exposure threshold for PrEP and selected a 60 mg oral once-monthly dose. A second oral presentation reported that an islatravir implant would provide PrEP cover for a year. [9]
At IAS 2021, PK data showed that drug levels using once-monthly oral PrEP remained at protective levels for at least two months after the last dose. Another study included plans to include islatravir in a vaginal ring, combined with a contraceptive. [14, 15]
Two large phase 3 studies are already underway using monthly oral islatravir. [16, 17]
If efficacy in these studies matches the animal data, islatravir as PrEP has the potential to eliminate HIV transmission. The potential as PEP as well as PrEP should make islatravir an over-the-counter medicine, likely matching blockbuster status similar to a statin or Viagra.
Lenacapavir (GS-6207) GS-CA1
Lenacapavir (previously GS-6207) is a first-in-class capsid inhibitor development by Gilead Sciences.
It is active at several stages of the viral lifecycle. It has picomolar activity with high potency (30–100 pM) and as a new class, is sensitive to HIV that is drug resistant to other classes including maturation inhibitors. It is given by 6-montly subcutaneous injection and being used for both treatment and prevention.
Lenacapavir for multidrug resistance
An application for treating MDR HIV has already been submitted to the US FDA, based on results from the CAPELLA (see pages 5–6 above).
Lenacapavir for treatment-naive
Early efficacy data from a phase 1b study was presented at the Glasgow 2020 conference. [1]
This study randomised 39 treatment-naive participants to a single dose of 20, 50, 150, 450 and 750 mg (n=6, 6, 6, 6 and 5) or placebo (n=10) as monotherapy. ART was started at day 10 (bictegravir/FTC/TAF) and follow-up continues to day 225.
Mean viral load responses at day 10, reported earlier this year at CROI, were dose-related and ranged from –1.3 log to –2.3 log in the 20 mg and 750 mg doses, respectively.
In vitro passaging studies have previously identified seven mutations associated with reduced sensitivity to lenacapavir: at positions L56I, M66I, Q67H, K70N, N74D, N74S and T107N in HIV capsid. But previous resistance studies found no pre-existing mutations in samples from 1500 treatment naive samples and 51 experienced samples retained wild-type sensitivity.
In this study, all participants had wild-type susceptibility to lenacapavir at baseline with no detection of capsid mutations.
However, at day 10, two participants showed Q67H at lowest doses – one each in the 20 mg and 50 mg groups, at day 10 and day 7 respectively. From the group listed above, this mutation has the lowest impact on reduced sensitivity (approximately 6-fold). No other substitutions were seen.
The 20 mg case showed Q67Q/H mix at day 10, and VL reduction continued after adding BIC/F/TAF.
The 50 mg case showed Q67H at day 7, detected only by the more sensitive next generation sequencing, but with evidence of a viral rebound in the few days before ART at day 10.
Both participants achieved undetectable viral load on ART.
The presentation also showed that drug resistance would be unlikely with the 300 mg and 600 mg doses selected for the phase 2/3 clinical programme.
As treatment, lenacapavir will be developed with other long-acting compounds including in a phase 1 study with two HIV bNAbs acquired from Rockefeller University (GS-5423 (teropavimab) and GS- 2872). [2, 3]
It will also be developed with Merck/MSD long-acting compounds, including islatravir. [4]
Other studies at CROI 2021 showed more details on the drug resistance profile including that in vitro mutations associated with reduced sensitivity, usually with reduced viral fitness (L56I, M66I, Q67H, K70N, N74D, N74S, and T107N) retained full sensitivity to HIV protease inhibitors darunavir and atazanavir. [5]
Another study, using X-ray crystal structures to report three distinct potential pathways for drug resistance, enabling research to develop second-generation molecules. [6]
A PK study reported AUC and Cmax concentrations that were 1.5- and 2.6-fold higher in mild to moderate hepatic impairment but that this would not require dose adjustment. [7]
IAS 2021 included results from the phase 3 CAPELLA study with extensive drug resistance (see report above) and from the phase 2 CALIBRATE study in treatment naive participants. [8]
CALIBRATE randomised 182 participants (2:2:2:1) to one of three lenacapavir arms with F/TAF (two using injections with later reduction to two-drug ART at week 28, one using oral lenacavir plus F/TAF throughout) or to a control arm of bictegravir/F/TAF.
Interim pre-specified 16-week results for achieving viral load <50 copies/mL included 92% (48/52), 94% (50/53), 94% (49/52), and 100% (25/25) in the three lenacapavir and control groups respectively.
Baseline characteristics included median age 29 years (range: 19 to 72), 7% women (yes, 7%), 52% black, 45% Hispanic/Latinx. Median viral load and CD4 count at baseline were 4.3 log copies/mL (IQR: 3.8 to 4.7) with 15% >100,000 copies/mL and 437 cells/mm3 (IQR: 332 to 599), with only two participants <200 cells/mm3.
At week 16 by ITT analysis, viral load was <50 copies/mL in 94% (147/157) vs 100% (25/25) in the pooled lenacapavir vs control groups respectively. The two cases of virological failure included one participant who did not reach <50 copies/mL at week 28 and one who discontinued the study after two days. Early response rates at week 4 were similar in all groups. The primary endpoint is at week 54.
One participant who had an early viral response at week 2 that rebounded close to 100,000 copies/mL baseline by week 10, developed lenacapavir emergent mutations (capsid Q67H + K70R) associated with a 20-fold loss of sensitivity, together with M184V in RT. Lenacapavir drug levels were consistently within the target range and although viral load was dropping again, treatment was changed to AZT/3TC/TDF plus dolutegravir (an unusual choice) and then became undetectable.
Adverse events were similar across groups (including 11 cases of COVID-19 and 17 cases of syphilis overall) with no drug-related discontinuations or grade 4 side effects.
Injection site reactions (ISRs) were common (40/183) but mostly grade 1 (33/40), with only 1 grade 3 and no grade 4. However, the study reported some nodules lasting for several months that were “palpable but not visible” and these extended from 1 to 4 cm. Two participants discontinued due to grade 1 ISRs with local hardening of the skin.
Laboratory abnormalities included high creatine kinase (n=5 vs 0), mainly explained by recent exercise with no grade 3/4 results judged clinically relevant or leading to discontinuations.
These results support continuing to the dual therapy maintenance therapy switches with extended follow-up to week 80.
Lenacapavir for PrEP
Lenacapavir is also being developed as PrEP. Results from macaque studies showed
96% (p=0.0002) and 100% (<0.0001) reduced risks of infection compared to placebo from both rectal and vaginal exposure, in studies reported at CROI 2021 and IAS 2021 respectively. [8, 9]
Phase 3 studies as PrEP are already planned and due to enrol shortly. [10]
Maturation inhibitors: GSK3640254 and GSK3739937
Maturation inhibitors work at the late stage of the viral lifecycle by producing non-infectious undeveloped HIV. As with other new classes, maturation inhibitors will not be cross-resistant to other types of HIV drugs.
GSK have two second-generation compounds in this class.
CROI 2021 included results on GSK3640254 from a phase 2a two-stage dose-finding study in 34 treatment-naive participants (n=6 per dose and n=2 placebo in each stage). Oral dosing was once-daily and given with a moderate fat meal. [1]
In stage 1, participants were randomised to either 10 mg of 200 mg for ten days. In part two, doses were 40 mg, 80 mg or 140 mg for seven days. Follow-up in stage 1 continued without treatment from days 11 to 17, with ART started on day 18. In stage 2, ART was started on day 8.
Mean age was 31, 94% were men and mean baseline viral load range from about 15,000 to 65,000.
Changes in viral load were roughly proportional to dose, with mean changes in plasma viral load ranging from −2.0 to 0.2 log copies/mL. The greatest mean reductions of –2.0 and –1.5 log were greatest in the 200 mg and 140 mg groups, respectively.
However, 4/6 participants in the 200 mg arm in stage 1 developed drug resistance at day 11 with A364A/V partial mixed variant which by day 21 had developed into the full mutation in 1/4 with 132-fold phonotypic resistance. No resistance was seen in the 10 mg arm but the results prompted the reduction to 7 days monotherapy in stage 2 (and where no drug resistance was reported).
Tolerability was good with all adverse events at grade 1 or 2 and no dose signal. The only two serious events (anal abscess and congestive cardiomyopathy) were not judged related to the study drug.
In a second study, GSK254 retained activity against a panel of clade B and C viruses with site directed mutations in gag (including V362I, V370A, Δ370, or R286K/V370A) that had limited activity of earlier maturation compounds, but showed a significant loss of sensitivity to A364V. [1]
Median EC50 values were 1.4 nM (range: 0.48 to 6.9 nM) and 1.4 nM (range: 0.85 to 1.9 nM) for Subtype B and C respectively and the study also reported in vitro studies clarifying the mechanism of action.
The phase 2b dose-finding DOMINO study, in 150 treatment-naive, will use 100 mg, 150 mg and 200 mg doses of GSL254 plus NRTIs, with the control group using dolutegravir-based triple therapy. [2]
A second phase 2 study will use the same three doses of GSK254 in dual combinations with dolutegravir, in 80 treatment-naive participants and using a control group of dolutegravir/lamivudine. [3]
GSK3739937 (VH3739937) is a second maturation inhibitor compound in development as a long acting injectable formulation (both subcutaneous and intramuscular). A phase 1 PK and safety study is ongoing. [4]
Both compounds are being developed by GSK/ViiV.
Albuvirtide – fusion inhibitor
Albuviride is an HIV fusion inhibitor that works at an early stage of the HIV lifecycle by blocking attachment to CD4 cells. It was approved in China in June 2018. [1]
It has a similar structure and mechanism to an earlier HIV fusion inhibitor called enfuvirtide (T-20, Fuzeon) that was developed for people who had run out of treatment options.
However, it is back in the pipeline report again because very limited results from the phase 3 TALENT study were presented at IAS 2021. This compared a two-drug arm with lopinavir/r was comparable to lopinavir/r with two NTRIs. [2]
Two US phase 2 studies are listed for use in multidrug resistance together with the bNAb 3BNC117. [3, 4]
A press release from Frontier Biotech reported approval in Equador. [5]
7. HIV bNAbs
Last year the pipeline report included at least a dozen broadly neutralising monoclonal antibodies (bNAbs) being studied for HIV treatment, prevention cure-related research. See Table 3.
These compounds have been isolated from people with strong long-term immune responses to HIV and engineered to improve potency and breadth of coverage often with the LS mutations to extend half-life/dosing times.
As with other antiretrovirals, bNAbs need to be used in combination to limit the risk of escape variants and drug resistance. This also requires baseline testing to confirm sensitivity before use. Current research is looking at their potential to generate immune responses in addition to their direct antiviral effect. And new delivery systems include the potential for the body to continue to produce further antibodies long-term.
This is complex field with new antibodies being discovered and two plenary talks at CROI in 2021 and 2019 are recommended as overviews. [1, 2]
So the main developments discussed below are not comprehensive, especially given the number of combinations in early stage research. These include news about VRC01, elipovimab, VRC07-523-LS, PGT121, 10-1074 and 3BNC117. For notes on other bNAbs please refer to the 2020 report. [3]
The 2021 HIV R4P conference held in January included a session on the HIV bNAb pipeline. Webcasts include a presentation on bispecific bNAbs including early viral loads results with 10E8.4/iMab (that uses ibalizumab). [4, 5, 6]
The three main clinical interventions in the last year include:
- Proof-of-concept for using bNAbs as adult prevention.
- Potential protection for infants during pregnancy and from breastfeeding.
- To maintain viral suppression off ART in cure-related strategies, sometimes with other immune modulators. anti-CCR5 humanized IgG4
Table 3: bNAbs for HIV prevention, treatment or cure research
| Compound / Company |
Target | Notes | Status |
| leronlimab (PRO 140).
CytoDyn. |
anti-CCR5 IgG4 bNAb | Once-weekly sub-cutaneous injection being studied in addition to ART for multi-drug resistance and as monotherapy maintenance therapy (without ART). Phase 3. | Phase 3. Submission to EMA by Nov 2021. |
| UB-421.
United BioPharma. |
CD4 binding | Infusion dosed either weekly or every two weeks as alternative to ART during treatment interruption. Phase 3. | Phase 3. |
| VRC01 and VRC01LS.
US NIH. |
CD4 binding | Intravenous infusion being studied in cure research and as PrEP. Two large phase 3 studies showed no overall benefit but proof of concept in a sub-group. Sub-cutaneous dosing of infants to prevent transmission at birth or from breastfeeding. VRC01LS is a longer acting formulation. | Phase 3. |
| VRC07, VR07-523LS | CD4 binding | Engineered from VRC01. Being studied with cabotegravir-LA in ACTG trial. | Phase 2. |
| PGT-121 and GS-9722 (elipovimab).
Gilead. |
C3/V3 | PGT121 is an IgG1 mAb that targets the V3 Env epitope. GS-9722 (elipovimab) is engineered from PGT-121. | Phase 1. |
| 3BNC117 and 10-1074.
Rockefeller University and Gilead. |
CD4 binding and C3/V3 | Both bNAbs are available as LS long-acting formulations.
Gilead Sciences signed for exclusive global development rights. |
Phase 2. |
| N6.
US NIH and ViiV. |
gp120 | Developed by US NIH and now licenced to ViiV. | Phase 1. |
| Other mAbs: 10E8, trispecific bNAbs, PGDM1400. | MPER, V2 and others. | Mulitple compounds in preclinical and phase 1 studies. | Phase 1. |
VRC01 as prevention in AMP studies
VRC01 was identified in 2009 in an HIV positive slow progressor in the US NIH cohort and isolated and manufactured into a treatment in a collaboration involving many leading public health laboratories.
Results from two large international prevention studies using VRC01 were represented at the R4P virtual conference in 2021. [7]
The Antibody Mediated Prevention (AMP) studies enrolled more than 4600 participants to receive VRC01 or placebo every two months over two years (total 10 infusions). Although the studies didn’t reduce HIV transmission overall, it produced 75% efficacy in a subset of participants exposed to virus that was sensitive to VRC01. This subgroup reported 9 vs 19 infections in the pooled VRC01 vs placebo arms respectively: prevention efficacy (PE) 75.4 % (95%CI: 44.5 to 88.9).
The lack of effect overall was related to several factors including dosing, formulation and use of mAb monotherapy, some of which were suspected before the studies started. However, the results provided essential proof-of-principle evidence that bNAbs can be an effective and acceptable treatment, including in low-income countries. It also showed how community engagement – an important part of this study – could enable and support recruitment and continued involvement in complicated prevention studies.
bNAbs as prevention during pregnancy and breastfeeding
Several research groups are also looking at the potential of long-acting bNAbs, including VRC01LS, for infant HIV prophylaxis and treatment.
A study at CROI 2021 modelled pooled PK data from HIV-exposed infants (IMPAACT P1112, n=21) and healthy adults (VRC606, n=49) to show that, in context of prophylaxis during pregnancy and during breastfeeding, 3-monthly dosing of VRC01LS should be sufficient in both adults and infants. [8]
Another study at CROI 2021 reported results when dual bNAbs were given to eight children who had undetectable viral load on early ART. [9]
IV infusions of VRC01LS and 10-1074 generated concentrations similar to those following single bNAb administration. Monthly dosing of VRC01LS at 15mg/kg and 10-1074 at 30 mg/kg achieved target concentrations at steady state. Safety was also good. Over 32 weeks follow-up there were no treatment related adverse events, and no grade 3 or 4 events.
Extended safety and PK results in HIV negative participants were reported for VRC07-523LS at the HIV R4P 2021 conference.
The ongoing HVTN127/HPTN087 study, using different doses of subcutaneous (SC) or intramuscular (IM) administration, reported safety results out to 122 weeks. [10]
Safety was also reported as prophylaxis in HIV-exposed infants, given within four days of birth, with subsequent dosing every three months for those exposed through breast milk. [11]
VRC07-523LS and PGT121 are being given in combination studies in the CAPRISA 012A adult prevention study. [12]
CAP256-VRC26.25LS, engineered from an antibody isolated from a South African woman with HIV subtype-C is in phase 1 prevention studies and that might be given even 4–6 months. Currently in phase 1 studies. [13]
bNAbs as strategies for remission and cure
Two bNAbs developed at Rockefeller University are already in several phase 1 an 2 studies looking at viral control off ART. 3BNC117 and 10-1074 are both formulated into long-acting LS variants. Both compounds were also acquired by Gilead in January 2020.
Earlier studies with maintained viral suppression off-ART for at least five months in 13/17 participants, sustained for more than 12 months in two people. [14]
This research is looking at the potential to enhance humoral and T-cell immune responses and that viral suppression can be maintained after antibody levels become undetectable in plasma (though these might persist in other tissues).
Several studies in participants on ART than include a treatment interruption. [15, 16, 17 ]
This includes the UK RIO study that is now enrolling participants after being put on hold for safety reasons during COVID-19.
A small phase 1 study is giving 3BNC117 and 10-1074 to ten HIV positive participants with detectable viral load who are not on ART – with a recommendation to start ART if virological response is suboptimal. [18]
Other bNAb research
Finally, other studies are using bNAbs together with other immune-mediated treatment treatments, using combination strategies for HIV remission. [19, 20]
The discovery and development of molecules also includes new delivery systems (including using AAV vectors) and DNA gene transfer (to programme B cells) to enable the recipient to reproduce these bNAb long-term. [21, 22]
Two studies from CROI 2020 are also important to reference.
A phase 1b reported results with elipovimab (GS-9722) in HIV negative and HIV positive individuals that support two-weekly dosing. It is an engineered variant of PGT121 that has also been studied in combination with the TLR7 agonist vesatolimod to target the reservoir in cure-related research. Elipovimab is now in development with Gilead. [23, 24]
Results were also presented from a dose escalation study of N6LS (GSK3810109A) in HIV negative people, which is now in phase 2 studies. N6LS is a bNAb that was isolated from an individual who was HIV positive for 21 years without ART and developed by the US National Institute of Allergy and Infectious Diseases (NIAID). It is broader and more potent than VRC01, neutralising up to 98% of viral strains and is now being developed by GSK. [25, 26, 27]
8. Other compounds
Although little data has been reported for the following compounds over the last year (at least), they are still in development.
This includes UB-421, elsulfavirine (long acting), ABX464 and BIT225. See Table 4.
Table 4: Compounds without new results
| Compound/ Company | Class | Notes | Phase |
| elsulfavirine (VM-1500). | NNRTI. | Developed by Viriom, already used in Russia as once daily oral drug. Long-acting formulation in development for monthly IM or SC injection. | Phase 2. |
| UB-421. | bNAb. | United BioPharma (Taiwan). | Phase 2. |
| ABX464. | Rev inhibitor. | Phase 2. | |
| BIT225. | VPU. | Phase 2. |
9. Discontinued compounds
The development of several compounds was discontinued over the last year.
These include the adnectin/fusion inhibitor combinectin (GSK3732394) with ViiV Healthcare and two tenofovir prodrugs (MK-8504 and MK-8583) with Merck/MSD.
Some of these had shown promising early results but the decision to stop further research was likely based on the profiles of other compounds now in development.
10. Conclusion
For all the challenges over the last year, the 2021 HIV pipeline has the potential to be more exciting than for many years.
If the promise of long-acting compounds holds out with clinical data, we are at the start of a new phase in the management of HIV treatment and prevention that could be as significant as the first years of ART, or the second stage that saw the development of 10 separate single pill fixed dose combinations.
But we need data. For all the potential advantages of long-acting formulations there remain uncertainties that need to be explored not just in phase 3 studies, but afterwards, to cover those who were not able to enrol. This includes older participants, use during pregnancy, preexisting renal and hepatic complications, cardiovascular disease, mental health issues and use of many as yet unstudied medications etc. [1]
If early drug resistance occurs, will the long dosing intervals increase the risk of accumulating mutations? How will this be affected by viral blipping that has been reported despite directly administered long-acting doses?
There might also be variability of the received dose depending on small differences in the way an injection is administered. Whether intramuscular injections are placed deep or in peripheral tissue, or if they miss the muscle completely. Whether the rate of release is constant throughout the dosing period, and whether any swelling afterwards affects drug release. [2]
But if this all works out well, then the next generation of ART might enable many people to use alternative treatments to daily oral pills that for many might approach a freedom similar to HIV remission.
Advances in pharmaceutical approaches to prevention – not the primary focus of this report (we have not covered dapivirine ring and related technology) – could largely end HIV transmission. This is even accepting that complex social and economic factors drive risk and health inequality, globally and within countries. [3, 4]
Within ten years – and also not the focus of this report – we might even have a therapeutic cure. [5]
11. Postscript updates
The following reports were made after the main report was published.
- CROI 2023: Six-monthly ART – lenacapavir + dual bNAbs maintains undetectable viral load for 26 weeks after single doses (Feb 2023)
- Glasgow 2022: pipeline studies on bNAb N6LS and maturation inhibitor GSK254 (October 2022)
- Glasgow 2022: doravirine updtes (October 2022)
- Glasgow 2022: long-acting cabotegravir/rilpivirine: adverse events, implementation and PROMs (October 2022)
- Discontinuation of the NNRTI MK-8507 due to reductions in total lymphocyte and CD4 T cell counts. (November 2021)
https://i-base.info/htb/41647 - Increased monitoring of islatravir studies for reductions in total lymphocytes and CD4 counts – both treatment and prevention. (December 2021)
https://i-base.info/htb/41647 - Selected islatravir studies stop enrolment: further complications with important investigational drugs. (December 2021)
https://i-base.info/htb/41833 - FDA further limit use of islatravir in ongoing studies. (December 2021)
https://i-base.info/htb/41866 - Long-acting cabotegravir/rilpivirine injections approved in the UK. (November 2021)
https://i-base.info/htb/41623 - Leronlimab (PRO 140) was submitted to EMA regulatory in November 2021.
https://www.io.nihr.ac.uk/report/leronlimab-in-addition-to-antiretroviral-therapy-for-treatment-experienced-adult-hiv-1-patients (download page)
https://www.io.nihr.ac.uk/wp-content/uploads/2021/07/17194-Leronlimab-in-addition-to-ART-for-HIV-Infection-V1.0-JULY2021-NON-CONF.pdf (PDF)
12. References
References to studies with clinical results are generally to earlier reports in HIV Treatment Bulletin (HTB). Direct links to the original source documents are included in these reports.
Abbreviations
CROI: Conference on Retroviruses and OIs. EMA: European Medicines Agency. FDA: Federal Drug Administration (USA). IAS: International AIDS Society. MDR: Multidrug Resistance. MHRA: Medicines and Healthcare Products Regulatory Agency (UK).
Introduction and viral lifecycle
- HIV i-Base. Pipeline report, March 2020.
https://i-base.info/htb/37221 - Barr L et al. Missing data, missing diversity: participant demographics in industry studies 2010-20. Poster abstract 418.
https://www.croiconference.org/abstract/missing-data-missing-diversity-participant-demographics-in-industry-studies-2010-20 - CROI 2021: HIV capsid uncoats in the CD4 nucleus rather than the cytoplasm – viral lifecycle updated… HTB (March 2021).
https://i-base.info/htb/40151 - Müller B et al. Live-cell imaging: capsid trafficking to the nucleus. CROI 2021. Oral abstract 19.
https://www.croiconference.org/abstract/live-cell-imaging-capsid-trafficking-to-the-nucleus - Li C et al. HIV-1 capsid retains its integrity until minutes before uncoating in the nucleus. CROI 2021. Oral abstract 01.
https://www.croiconference.org/abstract/hiv-1-capsid-retains-its-integrity-until-minutes-before-uncoating-in-the-nucleus
Cabotegravir/rilpivirine LA
- Long-acting injectable HIV treatment approved in the EU: includes two-monthly dosing. HTB (22 January 2021).
https://i-base.info/htb/39602 - Long-acting cabotegravir and rilpivirine injections support two-monthly dosing. HTB (12 March 2020).
https://i-base.info/htb/37301 - US FDA approves long-acting injectable HIV treatment: monthly dosing. HTB (22 January 2021).
https://i-base.info/htb/39697 - ViiV press statement. ViiV Healthcare Submits Supplemental New Drug Application to US FDA for Expanded Use of Cabenuva (cabotegravir, rilpivirine) as an HIV Treatment for Use Every 2-Months. (24 Fbruary 2021)
https://viivhealthcare.com/en-us/us-news/us-articles/2021/viiv-healthcare-submits-supplemental-new-drug-application-to-us-fd-for-expanded-use-of-cabenuva - Phase 3 results with dual therapy cabotegravir/rilpivirine long-acting injections: ATLAS and FLAIR studies. HTB: 20 (3). (12 March 2019).
https://i-base.info/htb/35812 - Orkin C et al. Long-acting cabotegravir + rilpivirine for HIV treatment: FLAIR week 96 results. CROI 2020. Poster 482.
https://www.croiconference.org/abstract/long-acting-cabotegravir-rilpivirine-for-hiv-treatment-flair-week-96-results - Jaeger H et al. Week 96 efficacy and safety of cabotegravir + rilpivirine every 2 months: ATLAS-2M. CROI 2021. Poster 401.
https://www.croiconference.org/abstract/week-96-efficacy-and-safety-of-cabotegravir-rilpivirine-every-2-months-atlas-2m - Benn P et al. Renal/bone outcomes after long-acting cabotegravir + rilpivirine in ATLAS + ATLAS-2M. CROI 2021. Poster abstract 541.
https://www.croiconference.org/abstract/renal-bone-outcomes-after-long-acting-cabotegravir-rilpivirine-in-atlas-atlas-2m - Cottura N et al. In silico prediction of long-acting cabotegravir PK in liver-impaired patients. CROI 2021. Poster abstract 374.
https://www.croiconference.org/abstract/in-silico-prediction-of-long-acting-cabotegravir-pk-in-liver-impaired-patients - Benn P et al. Long-acting cabotegravir+rilpivirine in older adults: pooled phase 3 week-48 results. CROI 2021, poster abstract 402.
https://www.croiconference.org/abstract/long-acting-cabotegravirrilpivirine-in-older-adults-pooled-phase-3-week-48-results - Rossenu S et al. POPPK modeling of Q2M IM RPV LA for managing dosing interruptions in HIV-1 patients. CROI 2021. Poster abstract 403.
https://www.croiconference.org/abstract/poppk-modeling-of-q2m-im-rpv-la-for-managing-dosing-interruptions-in-hiv-1-patients - Han K et al. Cabotegravir PPK simulation to inform Q2M strategies following dosing interruptions. CROI 2021. Poster abstract 373.
https://www.croiconference.org/abstract/cabotegravir-ppk-simulation-to-inform-q2m-strategies-following-dosing-interruptions - Patel P et al. Weight and lipid changes in phase 3 cabotegravir and rilpivirine long-acting trials. CROI 2021. Poster abstract 505.
https://www.croiconference.org/abstract/renal-bone-outcomes-after-long-acting-cabotegravir-rilpivirine-in-atlas-atlas-2m - Orkin C et al. Safety and efficacy of cabotegravir + rilpivirine long-acting with and without oral lead-in: FLAIR Week 124 results. Glasgow 2020.
https://vimeo.com/485456707/4e9da08e12 (webcast, starts at approx ! hour 3 minutes) - Orkin C et al. Week 124 results of the randomized, open-label, Phase 3 FLAIR study evaluating long-acting cabotegravir + rilpivirine for treatment in adults with HIV-1 infection (ITT-E population). IAS 2021. Oral abstract OAB0302.
https://theprogramme.ias2021.org/Abstract/Abstract/413 (abstract)
https://conference.ias2021.org/media-110-week-124-results-of-the-randomized–open-label–phase-3-flair-study-evaluating-long-acting (webcast) - Charpentier C et al. Prevalence of baseline virological risk factors of increased virological failure to CAB+RPV among ARV-naïve patients. IAS 2021. Oral abstract OAB0303.
https://theprogramme.ias2021.org/Abstract/Abstract/622 - Cabenuva. Full prescribing information (US).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212888s000lbl.pdf (PDF) - Long-acting cabotegravir injections are effective as HIV PrEP in gay men and transgender women: results from HPTN 083. HTB (July 2020).
https://i-base.info/htb/38534 - Two-monthly cabotegravir injections prevent HIV infection in African women: HPTN 084 study recommends early unblinding. HTB (November 2020).
https://i-base.info/htb/39327
Fostemsavir
- FDA approves fostemsavir (Rukobia) for multidrug resistant HIV in the US. HTB (22 July 2020).
https://i-base.info/htb/38355 - Fostemsavir approved in the EU (Rukobia): NICE deferred in the UK. HTB (22 January 2021).
https://i-base.info/htb/39703 - Fostemsavir: 96-week follow-up in people with multi-drug resistance. HTB (24 July 2019).
https://i-base.info/htb/36390 - Ackerman P et al. Clinical impact of antiretroviral agents used in optimized background therapy with fostemsavir in heavily treatment-experienced adults with HIV-1: exploratory analyses of the phase 3 BRIGHTE study. IAS 2021. Poster abstract PEB155.
https://theprogramme.ias2021.org/Abstract/Abstract/1961 - Shepherd B et al. Long-term (96-week) safety of fostemsavir (FTR) in heavily treatment-experienced (HTE) adults infected with multidrug-resistant (MDR) HIV-1 (BRIGHTE Phase 3 study). IAS 2021. Poster abstract PEB153.
https://theprogramme.ias2021.org/Abstract/Abstract/768 - EMA. Fostemsavir prescribing information.
https://www.ema.europa.eu/en/medicines/human/EPAR/rukobia - ClinicalTrials.gov. Low-dose fostemsavir extended release relative bioavailability study.
https://clinicaltrials.gov/ct2/show/NCT04757974
Paediatric dolutegravir
- FDA approves dolutegravir formulations to treat infants and young children. HTB (June 2020).
https://i-base.info/htb/38222 - EU approves dolutegravir 5 mg dispersible for children older than four weeks HTB (December 2020).
https://i-base.info/htb/39474 - Kityo C et al. Virological failures and genotypic resistance in children and adolescents randomised to dolutegravir-based ART vs. standard-of-care in the ODYSSEY trial. IAS 2021. Late breaking poster abstract PEBLB17.
https://theprogramme.ias2021.org/Abstract/Abstract/2446 - Lugemwa A et al. A randomised comparison of DTG-based ART vs standard of care in infants and young children living with HIV weighing 3 to 14kg: results from the ODYSSEY trial. IAS 2021. Late breaking poster abstract PEBLB18.
https://theprogramme.ias2021.org/Abstract/Abstract/2539 - Clayden P. Dolutegravir superior to standard-of-care in young children: results from the ODYSSEY trial. HTB (17 July 2021).
https://i-base.info/htb/40970 - Turkova A et al. Neuropsychiatric manifestations and sleep disturbances in children and adolescents randomised to dolutegravir-based ART vs standard-of-care in the ODYSSEY trial. IAS 2021. Oral abstract OAB0505.
https://theprogramme.ias2021.org/Abstract/Abstract/404 - Jacobs TG et al. No age-related difference in dolutegravir metabolic glucuronidation ratio in children between 3 months and 18 years old in the ODYSSEY trial. IAS 2021. Poster abstract PEB194.
https://theprogramme.ias2021.org/Abstract/Abstract/674 - Turkova A et al. Weight gain in children and adolescents on dolutegravir vs standard of care in the ODYSSEY trial. IAS 2021. Poster abstract PEB202.
https://theprogramme.ias2021.org/Abstract/Abstract/1311 - HIV i-Base. Paediatric pipeline report, 2021. (in press).
Lenacapavir – MDR indication
- Lenacapavir submitted to FDA as long-acting treatment for MDR HIV. HTB (July 2021).
https://i-base.info/htb/40859 - Gilead PR. European Medicines Agency validates Gilead’s marketing authorization application for lenacapavir, an investigational, long-acting capsid inhibitor for the treatment of HIV-1 in people with limited therapy options. (19 August 2021).
https://www.gilead.com/news-and-press/press-room/press-releases/2021/8/european-medicines-agency-validates-gileads-marketing-authorization-application-for-lenacapavir-an-investigational-longacting-capsid-inhibitor-for - CROI 2021: First results using capsid inhibitor lenacapavir against MDR HIV: potential for six-monthly ART and PrEP. HTB (April 2021).
https://i-base.info/htb/40290 - ClincalTrials.gov. Study to evaluate the safety and efficacy of lenacapavir in combination with an optimized background regimen in heavily treatment experienced participants living with HIV-1 infection with multidrug resistance.
https://clinicaltrials.gov/ct2/show/NCT04150068 - IAS 2021: lenacapavir studies show impressive results in naive, extensive drug resistance and potential as PrEP. HTB (July 2021).
https://i-base.info/htb/4100
Islatravir
- Merck acquires CMX157 and EFdA and starts phase 2 study for new NNRTI. HTB (October 2012).
https://i-base.info/htb/20318 - Orkin C et al. Week 96 analysis of viral blips from a phase 2b trial of islatravir and doravirine. CROI 2021. Poster abstract 416.
https://www.croiconference.org/abstract/week-96-analysis-of-viral-blips-from-a-phase-2b-trial-of-islatravir-and-doravirine - ClincalTrials.gov. Randomized, double-blind, efficacy, and safety study of doravirine/islatravir (DOR/ISL) in treatment-naïve participants with (HIV-1) Infection (MK-8591A-020)
https://clinicaltrials.gov/ct2/show/NCT04233879 - ClincalTrials.gov. Safety and efficacy of a switch to doravirine/islatravir in participants with HIV-1 (MK-8591A-017).
https://clinicaltrials.gov/ct2/show/NCT04223778 - ClincalTrials.gov. Switch to doravirine/islatravir (DOR/ISL) in HIV-1 participants treated with bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) (MK-8591A-018).
https://clinicaltrials.gov/ct2/show/NCT04223791 - ClincalTrials.gov. Open-label, follow-up of doravirine/islatravir for participants with HIV-1 infection (MK-8591A-033).
https://clinicaltrials.gov/ct2/show/NCT04776252 - ClincalTrials.gov. Doravirine/Islatravir (DOR/ISL) in Heavily Treatment-Experienced (HTE) Participants for Human Immunodeficiency Virus Type 1 (HIV-1) Infection (MK-8591A-019).
https://clinicaltrials.gov/ct2/show/NCT04233216 - ClincalTrials.gov. Doravirine/islatravir (DOR/ISL) in pediatric participants with HIV-1 who are <18 years of age and weigh ≥35 kg (MK-8591A-028)
https://clinicaltrials.gov/ct2/show/NCT04295772 - Dosing for once-weekly oral ART: islatravir plus MK-8507 studies due to start in 2021. HTB (12 March 2021).
https://i-base.info/htb/40158 - Single doses of MK-8507 reduce viral load by mean –1.5 log and support once-weekly dosing above 80 mg. HTB (14 October 2020).
https://i-base.info/htb/39085 - CROI 2021: Islatravir dosing for once-monthly and annual PrEP: if effective this could end HIV transmission. HTB (March 2021).
https://i-base.info/htb/40142 - Ankrom W et al. No pharmacokinetic interaction between novel NNRTI MK-8507 and Islatravir. IAS 2021. Poster abstract PEB171.
https://theprogramme.ias2021.org/Abstract/Abstract/1171 - Ankrom W et al. NNRTI MK-8507 does not alter the pharmacokinetics of the combined oral contraceptive levonorgestrel/ethinyl estradiol. IAS 2021. Poster abstract PEB169.
https://theprogramme.ias2021.org/Abstract/Abstract/688 - Hillier S et al. Safety and pharmacokinetics of oral islatravir once monthly for HIV pre-exposure prophylaxis (PrEP): week 24 analysis of a phase 2a trial. IAS 2021. Oral late breaker abstract OALC01LB03.
https://theprogramme.ias2021.org/Abstract/Abstract/2361 - Young I et al. Next generation 3D-printed intravaginal rings for prevention of HIV and unplanned pregnancy. IAS 2021/ Poster abstract 1PEC312.
https://www.ias2021.org/the-programme - ClincalTrials.gov. Oral Islatravir (MK-8591) once-monthly as preexposure prophylaxis (PrEP) in men and transgender women who have sex with men and are at high risk for HIV-1 infection (MK-8591-024).
https://clinicaltrials.gov/ct2/show/NCT04652700 - ClincalTrials.gov. Oral ISL QM as PrEP in cisgender women at high risk for HIV-1 infection (MK-8591-022).
https://clinicaltrials.gov/ct2/show/NCT04644029 - MSD/Merck stop once-weekly NNRTI MK-8507: islatravir studies continue with closer monitoring. HTB (19 November 2021).
https://i-base.info/htb/41647
Lenacapavir
- Capsid inhibitor lenacapavir, dosed six-monthly, has high barrier to drug resistance and no cross-resistance to other classes. HTB (October 2020).
https://i-base.info/htb/39080 - Gilead announces licensing agreement for Rockefeller University bNAbs. HTB (January 2020).
https://i-base.info/htb/37084 - ClincalTrials.gov. Study to evaluate the safety and efficacy of GS-5423 and GS-2872 in combination with lenacapavir (GS-6207) in virologically suppressed adults with HIV-1 infection.
https://clinicaltrials.gov/ct2/show/NCT04811040 - Gilead and Merck/MSD to collaborate on long-acting HIV combination of lenacapavir and islatravir. HTB (April 2021).
https://i-base.info/htb/40280 - VanderVeen L et al. Activity and resistance characterization of the HIV capsid inhibitor lenacapavir. CROI 2021. Oral abstract 128.
https://www.croiconference.org/abstract/activity-and-resistance-characterization-of-the-hiv-capsid-inhibitor-lenacapavir - Bester SM et al. Structural basis for viral resistance to long-acting HIV-1 capsid inhibitor GS-6207. CROI 2021. Poster 420.
https://www.croiconference.org/abstract/structural-basis-for-viral-resistance-to-long-acting-hiv-1-capsid-inhibitor-gs-6207 - Jogiraju v et al. Pharmacokinetics of lenacapavir, an HIV-1 capsid inhibitor, in hepatic impairment. CROI 2021. Poster 375.
https://www.croiconference.org/abstract/pharmacokinetics-of-lenacapavir-an-hiv-1-capsid-inhibitor-in-hepatic-impairment - IAS 2021: lenacapavir studies show impressive results in naive, extensive drug resistance and potential as PrEP/
https://i-base.info/htb/41003 - Bekerman E et al. Long-acting HIV capsid inhibitor effective as PrEP in a SHIV rhesus macaque model. CROI 2021. Poster 717.
https://www.croiconference.org/abstract/long-acting-hiv-capsid-inhibitor-effective-as-prep-in-a-shiv-rhesus-macaque-model - ClincalTrials.gov. Study to assess the effectiveness and safety of lenacapavir for HIV pre-exposure prophylaxis.
https://clinicaltrials.gov/ct2/show/NCT04925752
GSK254 maturation inhibitor
- Once-daily GSK254 maturation inhibitor as treatment for HIV multidrug resistance. HTB (March 2021).
https://i-base.info/htb/40180 - ClincalTrials.gov. A dose-range finding clinical trial study in HIV-1 infected treatment-naive adults.
https://clinicaltrials.gov/ct2/show/NCT04493216 - ClincalTrials.gov. A clinical trial of GSK3640254 + dolutegravir (DTG) in HIV-1 infected treatment-naive adults
https://clinicaltrials.gov/ct2/show/NCT04900038 - ClincalTrials.gov. GSK3739937 first-time-in-human (FTIH) study in healthy volunteers.
https://clinicaltrials.gov/ct2/show/NCT04493684
Albuvirtide
- China approves albuvirtide: a once-weekly injectable entry inhibitor. HTB (June 2018).
https://i-base.info/htb/34319 - Dai L et al. Efficacy and safety of long acting HIV fusion inhibitor albuvirtide in treatment-experienced HIV-1 infected patients: week 48 analysis from the randomized controlled phase 3 TALENT study. IAS 2021. Abstract PEB148.
https://conference.ias2021.org/media-52-efficacy-and-safety-of-long-acting-hiv-fusion-inhibitor-albuvirtide-in-treatment-experienc - Albuvirtide in combination with 3BNC117 in patients with multi-drug resistant (MDR) HIV-1 Infection.
https://clinicaltrials.gov/ct2/show/NCT04560569 - Albuvirtide in combination with 3BNC117 in virologically suppressed subjects with HIV-1 infection.
https://clinicaltrials.gov/ct2/show/NCT04819347 - Press release. Frontier Biotechnologies’ First Long-acting Injectable (Aikening(R)), in a Two Drug Regimen for HIV, Proves Safe and Efficacious for Patients. (19 July 2021).
https://www.prnewswire.com/news-releases/frontier-biotechnologies-first-long-acting-injectable-aikening-r-in-a-two-drug-regimen-for-hiv-proves-safe-and-efficacious-for-patients-813835556.html
bNAbs
- Caskey M. HIV-1 bNAbs: LOOKING AHEAD. CROI 2021. Oral abstract 36.
https://www.croiconference.org/abstract/hiv-1-bnabs-looking-ahead - Nussenzweig M. discovery and development of HIV broadly neutralizing antibodies. CROI 2019. Oral abstract 10.
http://www.croiwebcasts.org/console/player/41037 - HIV i-Base. Pipeline report, March 2020.
https://i-base.info/htb/37221 - Coming soon to a clinic near you? The antibody infusion pipeline. HIV R4P 2021.
https://programme.hivr4p.org/Programme/Session/34 - Sobieszczyk M. Engineered bispecific bNAbs. HIV R4P 2021. RT01.02
https://programme.hivr4p.org/Programme/Session/34 - ClincalTrials.gov. 10E8.4/iMab bispecific antibody in HIV-uninfected and HIV-infected adults.
https://clinicaltrials.gov/ct2/show/NCT03875209 - VRC01 antibody only prevents minority of HIV infections: AMP study results. HTB (24 February 2021).
https://i-base.info/htb/39977 - Yang J et al. Population pharmacokinetics of VRC01LS in term infants and adults. CROI 2021.
https://www.croiconference.org/abstract/population-pharmacokinetics-of-vrc01ls-in-term-infants-and-adults - Capparelli E et al. Safety and pharmacokinetics of VRC01LS and 10-1074 among children in Botswana. CROI 2021. Poster abstract 609.
https://www.croiconference.org/abstract/safety-and-pharmacokinetics-of-vrc01ls-and-10-1074-among-children-in-botswana - Walsh S et al. Safety and single-dose pharmacokinetics of VRC07-523LS administered via different routes and doses. R4P 2021.
https://programme.hivr4p.org/Abstract/Abstract/799 - Cunningham C et al. Safety and PK of potent anti-HIV monoclonal AB VRC07-523LS in HIV-exposed infants. HIVR4P 2021. Oral abstract OA0302.
https://programme.hivr4p.org/Abstract/Abstract/363 - Mahomed S et al. Assessing the safety and pharmacokinetics of the monoclonal antibodies, VRC07-523LS and PGT121 in HIV negative women in South Africa: study protocol for the CAPRISA 012A randomised controlled phase I trial. BMJ Open 2019;9:e030283. doi: 10.1136/bmjopen-2019-030283.
https://bmjopen.bmj.com/content/9/7/e030283.citation-tools - Doria-Rose N et al. Isolation of new CAP256-VRC26 lineage members reveals determinants of breadth and potency. HIVR4P 2021. PU0203.
https://programme.hivr4p.org/Abstract/Abstract/1253 - Dual bNAb maintains viral suppression for median 21 weeks off-ART. HTB (November 2018).
https://i-base.info/htb/35248 - ClincalTrials.gov. 3BNC117 and 10-1074 in ART-treated Individuals.
https://clinicaltrials.gov/ct2/show/NCT03526848 - ClincalTrials.gov. Evaluating a combination of immune-based therapies to achieve a remission of HIV infection (HIVACAR)
https://clinicaltrials.gov/ct2/show/NCT03619278 - ClincalTrials.gov. A randomised placebo controlled trial of ART plus dual long-acting HIV-specific broadly neutralising antibodies (bNAbs). (RIO)
https://clinicaltrials.gov/ct2/show/NCT04319367 - ClincalTrials.gov. 3BNC117-LS and 10-1074-LS in viremic HIV-positive Individuals.
https://clinicaltrials.gov/ct2/show/NCT04250636 - ClincalTrials.gov. Safety, tolerability, and efficacy of IL-15 superagonist (N-803) with and without combination broadly neutralizing antibodies to induce HIV-1 control during analytic treatment interruption.
https://clinicaltrials.gov/ct2/show/NCT04340596 - ClincalTrials.gov. Combinatorial therapy with a therapeutic conserved element dna vaccine, MVA vaccine boost, TLR9 agonist and broadly neutralizing antibodies: a proof-of-concept study aimed at inducing an HIV remission.
https://clinicaltrials.gov/ct2/show/NCT04357821 - Casazza J et al. Durable hiv-1 antibody production in humans after AAV8-mediated gene transfer. CROI 2021. Oral abstract 160.
https://www.croiconference.org/abstract/durable-hiv-1-antibody-production-in-humans-after-aav8-mediated-gene-transfer-2 - Jefferys R. A step forward for AAV-mediated delivery of bNAbs. HTB (April 2020).
https://i-base.info/htb/37604 - Safety and PK of bNAb elipovimab (GS-9722) support two-weekly dosing. HTB (March 2020).
https://i-base.info/htb/37310 - Moldt B et al. Evaluation of bNAb sensitivity by genotyping and phenotyping for HIV clinical trials. CROI 2021 Poster 425.
https://www.croiconference.org/abstract/evaluation-of-bnab-sensitivity-by-genotyping-and-phenotyping-for-hiv-clinical-trials - Widge AT et al. A phase I dose-escalation trial of human monoclonal antibody N6LS in healthy adults. CROI 2020, Boston. Poster abstract 508.
https://www.croiconference.org/abstract/a-phase-i-dose-escalation-trial-of-human-monoclonal-antibody-n6ls-in-healthy-adults - ClincalTrials.gov. A study to evaluate the antiviral effect, safety and tolerability of GSK3810109A in viremic HIV-1 positive adults.
https://clinicaltrials.gov/ct2/show/NCT04871113 - ClincalTrials.gov. VRC 609 Study: A phase 1, open-label, dose-escalation study of the safety and pharmacokinetics of a human monoclonal antibody, VRC-HIVMAB091-00-AB (N6LS), administered intravenously or subcutaneously with or without recombinant human hyaluronidase PH20 (rHuPH20) to HIV negative adults.
https://clinicaltrials.gov/ct2/show/NCT03538626
Conclusion
- Khoo S. Long-acting ARVs: Research into the pharmacology of treatment and prevention. HANC HIV AIDS Network. (30 April 2021).
https://fredhutch.hosted.panopto.com/Panopto/Pages/Viewer.aspx?id=6ef6e731-aaa6-46dd-b921-ad66016ddc60 - Dayananda et al. Intended intramuscular gluteal injections: Are they truly intramuscular? J Postgrad Med. 2014:60.175-8. DOI:10.4103/0022-3859.132334.
https://pubmed.ncbi.nlm.nih.gov/24823517 - EMA supports use of dapivirine vaginal ring to prevent HIV in high-incidence countries. HTB (20 August 2020).
https://i-base.info/htb/38818 - Jefferys R. PrEP and microbicides. TAG pipeline report 2021.
https://www.treatmentactiongroup.org/wp-content/uploads/2021/07/pipeline_2021_hiv_PrEP_final.pdf (PDF) - Jefferys R. Research Toward a Cure and Immune-Based Therapies. TAG pipeline report 2021.
https://www.treatmentactiongroup.org/wp-content/uploads/2021/07/pipeline_2021_hiv_cures_final.pdf (PDF)
This article was first published on 22 August 2021. The conclusion was updated in final publication to expand on PK considerations with long-acting formulations. Information on MK-8507 was updated on 14 November 2021.