2017: a year in review – great news and why we still fight…
5 January 2018. Related: Special reports.
 Polly Clayden and Simon Collins, HIV i-Base
Polly Clayden and Simon Collins, HIV i-Base
The following review uses hyperlinks in the text to articles from HIV Treatment Bulletin (HTB) in 2017.
Great news is always good to report…
Steady advances reported in HTB in 2017 were broadly categorised as great news. These are important given the political change and uncertainty over the year.
There was good news both within the UK and globally that will affect the majority of HIV positive people – especially the growing acceptance that undetectable viral load makes HIV untransmittable (U=U) and the impact of better treatment with new drugs and formulations and the wider use of PrEP. Together with earlier use of ART and more regular testing, many countries and regions are meeting some or all of the 90:90:90 goals and HIV incidence has dropped significantly when all these advances were available and easy to access.
We also reported on progress towards a cure and a vaccine, on new treatment strategies, and on complications linked to current treatment and side effects.
But in the interest of balance, we also had to report alternative good news (also known as bad news…) – showing that that none of the advances can be taken for granted. Overcoming AIDS is not inevitable unless we make it so.
Treatment access
The big news for global HIV treatment was a new pricing agreement that will speed up access to generic, dolutegravir-based fixed dose combinations (FDCs).
This will mean that HIV positive people in generic-accessible low- and middle-income countries (LMICs), can be treated at an annual cost per person of around US $75. The price announcement was made on 21 September 2017.
The FDCs combine tenofovir disoproxil fumarate, lamivudine, and dolutegravir (TLD) and were developed by Mylan and Aurobindo under licensing agreements from ViiV Healthcare. Both generics received tentative approval from the US FDA for TLD in August.
The FDCs will help enable countries to transition to DTG-based regimens. As of the end of the year, almost 60 countries had adopted or are planning to include DTG in national treatment guidelines for first-line ART. Brazil, Botswana, Kenya and Uganda have already started treating people with DTG. PEPFAR has recommended rapid introduction of DTG in their key target countries. It has been estimated that approximately 15 million people will be taking DTG by 2025 and it will replace first-line efavirenz.
This year DTG (as well as PrEP) was added to WHO essential medicines list.
And the country with the highest national prevalence in the world, Swaziland, with 32% among a population of just under 1.5 million in 2011, using currently recommended ART, saw a decrease in HIV incidence by almost half and a doubling of viral load suppression among adults, between 2011 and 2016.
The new DTG-based regimens will help more countries to provide ART to more people and get nearer to 90-90-90 targets.
HIV transmission: UK response
Closer to home, the year began with remarkable news on the 40% drop in HIV diagnoses at central London clinics, confirmed throughout the year.
The annual UK surveillance reports consolidated this phenomenon, showing similar percentage reductions in all seven regions of the UK.
The growing confidence that undetectable viral load means HIV is untransmittable (U=U) resulted in breakthrough endorsements, most crucially, from the US Centers for Disease Control and Prevention (CDC). A special report from i-Base reviewed the evidence and the 20-year timeline that it took to reach this stage, including new results from the Opposites Attract study.
Antiretrovirals: approvals and pipeline
During 2017, there were several approvals of new drugs and formulations, and many other compounds are still in development.
At the end of the year, Symtuza (darunavir/cobicistat/FTC/TAF aka D/C/F/TAF) was approved for adults and adolescents in the EU, the first single pill protease inhibitor-based FDC.
Similarly, Juluca (dolutegravir/rilpivirine) was approved in the US as a dual therapy switch option for adults on stable ART (undetectable viral load for at least six months). The indication includes not having previous treatment failure of drug resistance to the individual components. The EU decision is expected in early 2018.
Bictegravir was also submitted in the US with accelerated review. [13]
CROI 2017 produced results for bictegravir (phase 2), doravirine (phase 3), and GS-9131 (preclinical).
IAS 2017 included results on once-daily raltegravir (96 week phase 3), the bictegravir FDC (phase 3), doravirine (48 week phase 3), long-acting cabotegravir/rilpivirine injections, D/C/F/TAF (phase 3) and MK-8591 (early results).
EACS 2017 included results for fostemsavir (phase 3), D/C/F/TAF (phase 3).
Developments in paediatric ART included the FDA approval of raltegravir for treatment of neonates from birth to four weeks of age – weighing at least 2 kg. This approval was supported by data from IMPAACT P1110, looking at the safety and pharmacokinetics of raltegravir oral suspension in high-risk HIV exposed newborns. Raltegravir is now one of the few antiretrovirals approved for treating babies from birth.
Data presented at CROI 2017 revealed that tenofovir alafenamide (TAF) exposure is modestly higher in children aged 6–12 years than adults.
Another CROI presentation showed DTG granules-in-suspension achieved satisfactory exposures in children of the same age. This formulation will not be commercially available but these data will form the basis for DTG dosing as dispersible tablets to be studied in this and younger age (and weight-based) cohorts, which are now enrolling.
And a presentation at the 9th International Workshop on HIV Pediatrics 2017 showed chewable raltegravir tablets can be crushed and stirred until dispersed in liquids and given to younger children according to WHO weight bands.
Fit for Purpose, our twice-yearly review of developments in ART optimisation expanded in July to include our review of the adult pipeline (a comprehensive summary of 25 compounds) as well as paediatric developments.
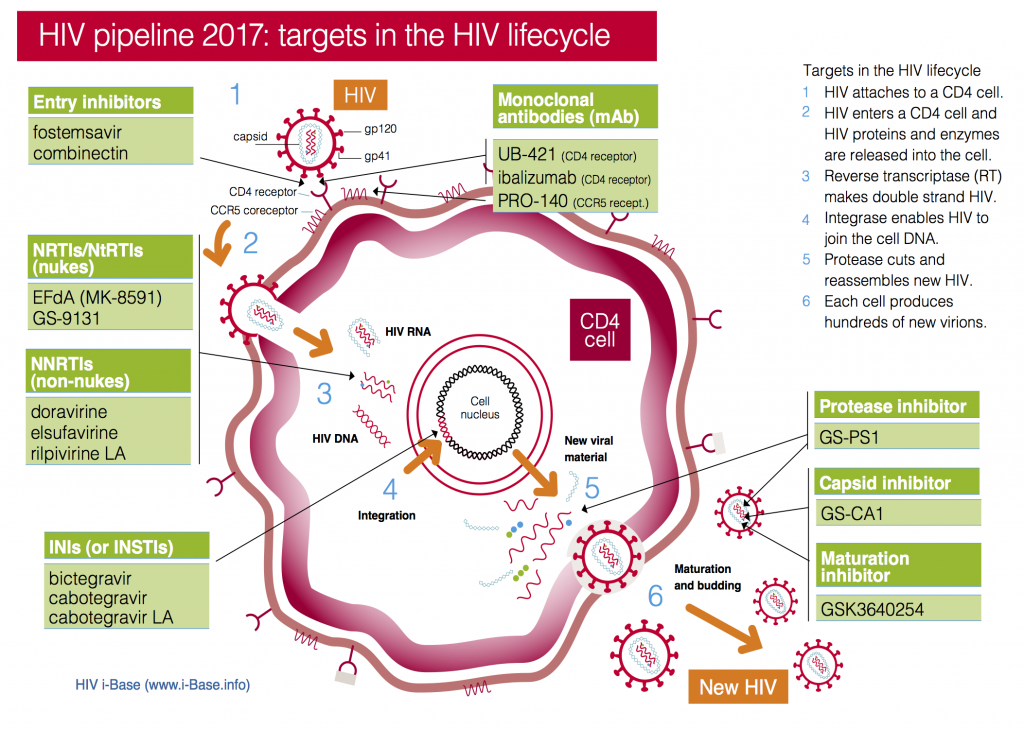
We also produced a more detailed HIV pipeline review.
Figure 1: HIV pipeline 2017: targets in the HIV lifecycle
Key: INSTI: Integrase strand transfer inhibitors; NRTI: Nucleoside/tide reverse transcriptase inhibitors; NNRTI Non-nucleoside reverse transcriptase inhibitors.
Treatment strategies
Almost as quickly as the first reports appeared of using dolutegravir monotherapy the risks of this approach were quickly discovered.
The plausibility that this single drug might hold back resistance surprisingly maintained viral load at undetectable levels for a large percentage of people. But for those with viral rebound – highly unpredictable – the cost was high-level integrase resistance, and future loss of arguably the most important current drug class. No-one should now be using dolutegravir monotherapy.
Neither of these problems have so far been reported in the ongoing dual therapy studies using dolutegravir plus 3TC. These include both as first-line ART and numerous studies as a switch option. But this strategy – the focus of ongoing two phase 3 GEMINI studies – does have the potential to improve quality of life from treatment simplification – and so the research is important.
Another much-discussed strategy of whether anti-inflammatory drugs might have a role in addition to ART will be informed by long-awaited results from the CANTOS canakinumab study. But while the accumulating research linking HIV to ongoing immune inflammation – even on ART – suggest an easy target, many of the prospective drugs studies so far have produced disappointing results.
Pregnancy and breastfeeding
One of the reasons that DTG is an alternative and not a preferred option in WHO guidelines was the lack of data in pregnant women.
So, it was reassuring that reports presented at IAS 2017 on DTG use in pregnancy from Botswana, Europe and the Antiretroviral Pregnancy Registry, did not show an increased risk of adverse outcomes compared with other antiretrovirals.
But more data are needed, particularly with DTG exposure before conception, to reach definitive conclusions – and we expect that to be forthcoming in 2018 and a guideline change is likely.
Other important findings in pregnant women this year included:
- Maternal ART of efavirenz, tenofovir and FTC – the current WHO preferred regimen – was associated with lower risk of adverse birth outcomes compared with other regimens, among infants exposed to ART from conception in Botswana.
- Women receiving lopinavir/ritonavir-based regimens were at higher risk of preterm delivery compared with those on NNRTI-based ones in UK and Ireland.
- A pharmacokinetic study of 400 mg efavirenz during pregnancy, showed lower drug concentrations in the third trimester, compared with post-partum, but these were within adequate ranges described elsewhere – so the reduced dose could be used in pregnant women.
And good news from Tanzania on breastfeeding was that no HIV exposed infants who were negative at birth, whose mothers started ART before delivery, had suppressed viral loads and exclusively breastfed, were HIV positive after breastfeeding, in a rural cohort.
Basic science
Throughout the year, Richard Jefferys produced a consistently impressive series of reports on developments on basic science, cure and vaccine research.
This included challenges of identifying markers for the cellular reservoir, estimating the size of the viral reservoir, remission news from IAS 2017, and a compelling update – the Miami macaque – on vaccine research.
These were supported by similar in depth reports by Gareth Hardy, including on CD163 as a marker of immune activation and CMV antibody as a marker of HIV progression. Plus other reports on rosuvastatin for COPD, and kidney disease.
Alternative good news (aka bad news…)
Two Cochrane reviews: low points.
Most issues of HTB include i-Base reviews of key publications in peer-review journals. While most of our reports from conferences bring important research to readers well in advance of publication, the consolidation of full results in journals is still essential.
Unfortunately, this year included two papers that i-Base (and many others) promptly and publicly criticised – almost before the ink had dried in the email alerts. Both papers used problematic methodology to reach flawed conclusions.
These attempts to generate headlines are not merely academic – they can have serious consequences.
Our review of the BMJ paper on the safety of HIV drugs during pregnancy highlighted both the lack of evidence for claims to challenge WHO guidelines and the potential destabilising impact this would have on the majority of HIV positive women of child bearing age throughout the world dependent on currently recommended ART.
We also highlighted problems in a Cochrane review of new hepatitis C drugs which, by asking inappropriate questions, risked removing pressure to provide these life-saving new drugs.
UK access to PrEP
Each year PrEP makes headlines and 2017 was no exception. In the UK, much of this focus was on access (and lack of).
While the NHS now provide free PrEP in Scotland and in Wales, in England, the controversial PrEP IMPACT Study has started to enrol as the only way to currently get free PrEP. As with Wales and Scotland, this includes broadening access beyond gay and bisexual men. However, with many of the first sites fully enrolled almost as soon as they opened, and others taking longer to start, access problems are likely to continue.
IPERGAY provided important additional results on the efficacy of on-demand dosing for anal sex from both the expanded access study and for men having less frequent sex. This is only an option when the risk is only from anal sex.
We also reported the psychological benefits from PrEP.
EMA move to Amsterdam
One of the many unforeseen consequences of Britain’s 23 June 2016 act of self-harm will be the relocation of the EMA to Amsterdam.
The agency has been based in London since its establishment in 1995. It currently employs almost 900 staff at its headquarters in Canary Wharf. Now it has been forced to relocate by the end of March 2019, and the UK will be much poorer for it.
UK drug approval post-Brexit is now currently unknown, as we clearly don’t have the resources to evaluate new drugs on an individual country level. Although the government has stated it wishes to work closely with the EMA in regulating medicines for the UK market and global drug companies are in favour of some form of cooperation, it will be up to EU member states to decide how this will work.
The relationship with the Medicines Health and Regulatory Agency (MHRA), which regulates UK drugs and healthcare products, will have to change. MHRA has had a lucrative working arrangement with the EMA and has carried out 20 to 30% of the licensing work for the agency.
This change is not only going to be costly but likely to result in delays in approvals to new medicines for British patients compared with those in EU countries.
Proposals to relax evidence requirements for drug approval
Another major concern over the last year has been a shift to reduce regulatory requirements for the approval process of new medicines.
Disturbingly, the publicity supporting lobbying for such changes is frequently presented as the result of community demand for faster access to medicines. Actually, the opposite is more likely to be true. The lobbying – and the great expense behind it – is closely connected to the financial interests of large pharmaceutical companies.
In December 2016, the US Congress passed the 21st Century Cures Act making the demands for new drug approval less strict and more flexible. More specifically, this act aims to reduce the role of large randomised clinical studies and allows companies to sell products supported by less evidence to patients cloaked as a “right to try” access. In turn, companies could also use so-called “real world” or even anecdotal reports to support full drug approval.
In Europe, similar lobbying led to the EMA proposing the Adaptive Pathways approach, which set out to enable circumstances for faster drug approval – although none of the 62 compounds submitted in the pilot phase led to successful faster approval.
Vulnerable, entitlement, diversity, transgender, foetus, evidence-based, science-based
Finally, more alarming news from the Trump administration.
In December, the US CDC was instructed that words and phrases including the above, were to be banned from use in budget documents.
It is important that the CDC denied that they would follow such restrictions. Similar resistance needs to become universal.
Striking out words is like striking out people. More than ever, this is a time to make our networks of friendships stronger.
i-Base news and publications
Conference reports
Although many of these articles will have been referred to in the summary above, i-Base continued to report on hundreds of studies in comprehensive reviews from at least eight major HIV conferences and workshops.
All coverage is linked to the holding page for each of these meetings:
- CROI 2017
- BHIVA 2017
- HIV PK workshop
- 11th INTEREST Workshop
- 9th International Paediatric Workshop
- IAS 2017
- EACS 2017
- 8th HIV and Ageing.
Resources
 Running alongside HTB, i-Base produces non-technical resources for HIV positive people.
Running alongside HTB, i-Base produces non-technical resources for HIV positive people.
During 2017 this included an expanded range of pocket-size concertina leaflets, updated guides to PrEP in the UK, changing HIV treatment, a summarised guide to PrEP in Scotland, Trans health, Pregnancy in Swahili…
Also a 32-page A4 publication ART in pictures: HIV treatment explained which was quickly translated in Russian.
Funding appeal
 The i-Base appeal was launched in April to support our projects that are increasingly vulnerable due to changes in financial support.
The i-Base appeal was launched in April to support our projects that are increasingly vulnerable due to changes in financial support.
i-Base continues to be the only HIV organisation that provides all patient resources free to NHS clinics – the only ethical model to match the NHS principal of free at point of care access.
We have done this for 17 years, generally running on a tight budget and without the luxury of reserves.
Please become an active supporter if you are able to help these services during 2018.
Conclusion
Altogether, 2017 was a lively year.
There is every prospect that many of the trends reported above will continue into 2018.
We hope you join us to realise and build on the positive news and similarly help to highlight ways to make the fight for the best quality health care in the UK and globally.